Question: 65. Doubling the initial concentration of a reactant doubles t1/2 of the reaction then order of reaction is :- (1) 3 (2) 2 (3)

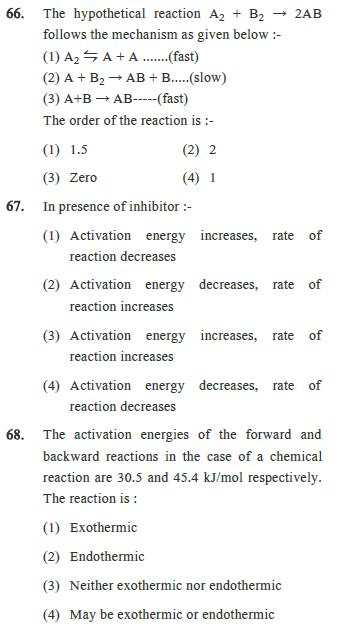

65. Doubling the initial concentration of a reactant doubles t1/2 of the reaction then order of reaction is :- (1) 3 (2) 2 (3) 1 (4) Zero 66. The hypothetical reaction A2 + B2 2AB follows the mechanism as given below:- (1) AA+A.......(fast) (2) A + B AB + B.....(slow) (3) A+B AB-----(fast) The order of the reaction is :- (1) 1.5 (3) Zero (2) 2 (4) 1 67. In presence of inhibitor :- (1) Activation energy increases, rate of reaction decreases (2) Activation energy decreases, rate of reaction increases (3) Activation energy increases, rate of reaction increases (4) Activation energy decreases, rate of reaction decreases 68. The activation energies of the forward and backward reactions in the case of a chemical reaction are 30.5 and 45.4 kJ/mol respectively. The reaction is: (1) Exothermic (2) Endothermic (3) Neither exothermic nor endothermic (4) May be exothermic or endothermic 69. 75% of a 1st order reaction was found to complete in 32 minute. When will 50% of the same reaction will complete :- (1) 32 min (2) 16 min (3) 64 min (4) 8 min
Step by Step Solution
There are 3 Steps involved in it
65 If doubling the initial concentration of a reactant doubles t... View full answer

Get step-by-step solutions from verified subject matter experts


