Question: 7. Differences in boiling point between different compounds can be explained by examining the types of intermolecular forces that exist between molecules. Explain the trend
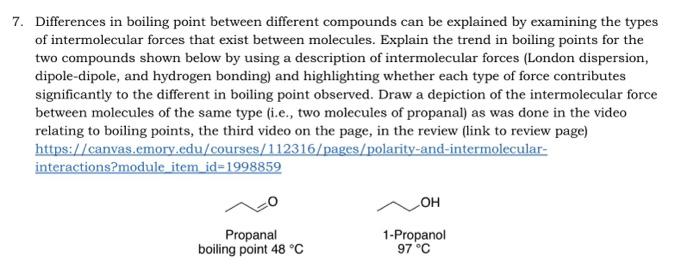
7. Differences in boiling point between different compounds can be explained by examining the types of intermolecular forces that exist between molecules. Explain the trend in boiling points for the two compounds shown below by using a description of intermolecular forces (London dispersion, dipole-dipole, and hydrogen bonding) and highlighting whether each type of force contributes significantly to the different in boiling point observed. Draw a depiction of the intermolecular force between molecules of the same type (i.e., two molecules of propanal) as was done in the video relating to boiling points, the third video on the page, in the review (link to review page) https://canvas.emory.edu/courses/112316/pages/polarity-and-intermolecular
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


