Question: 7. Nickel has a face-centered cubic structure and has a density of 8.90g/cm3. What is the volume of the unit cell in cm3 ? Report
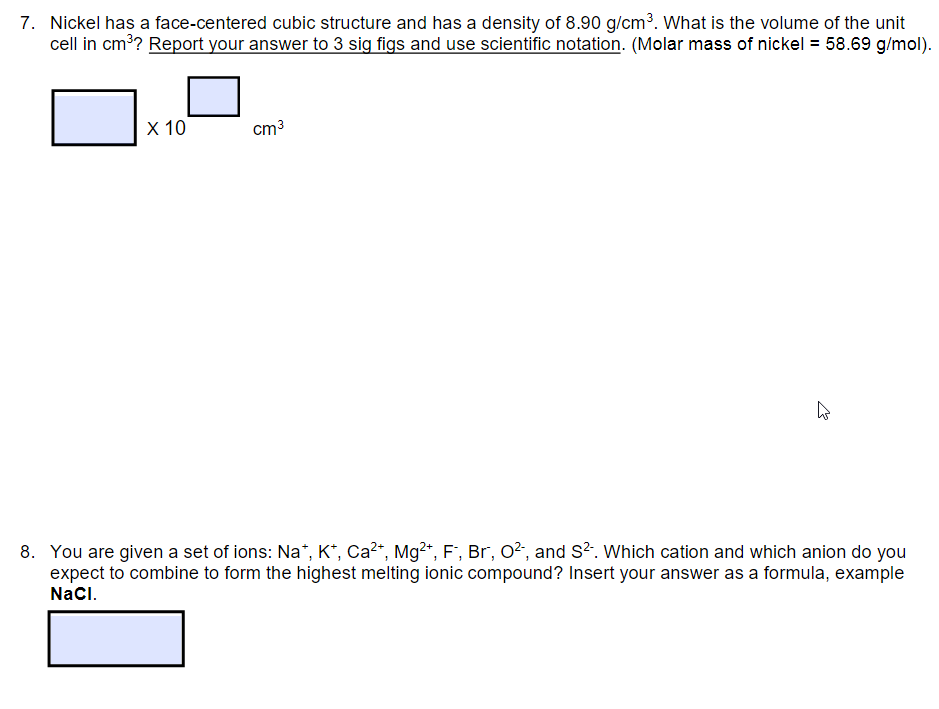
7. Nickel has a face-centered cubic structure and has a density of 8.90g/cm3. What is the volume of the unit cell in cm3 ? Report your answer to 3 sig figs and use scientific notation. (Molar mass of nickel = 58.69g/mol ). 8. You are given a set of ions: Na+,K+,Ca2+,Mg2+,F,Br,O2, and S2. Which cation and which anion do you expect to combine to form the highest melting ionic compound? Insert your answer as a formula, example NaCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


