Question: 7. Predict the missing value (?) for radioactive francium (Fr). The atomic radius, density, and melting point are given for elements in Group IA/1. 1.
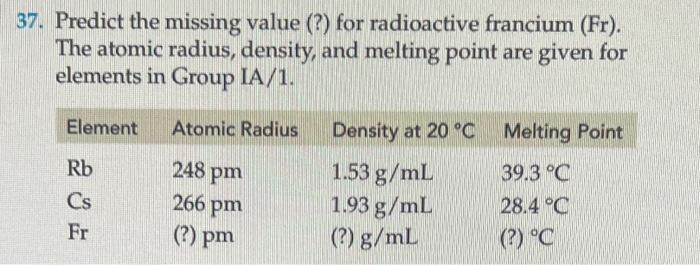

7. Predict the missing value (?) for radioactive francium (Fr). The atomic radius, density, and melting point are given for elements in Group IA/1. 1. The formulas for the chlorides of potassium, calcium, boron, and germanium are, respectively, KCl,CaCl2,BCl3, and GeCl4. Using the periodic table, predict the chemical formulas for each of the following similar compounds. (a) potassium fluoride (b) calcium fluoride (c) boron bromide (d) germanium iodide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


