Question: 7.(1) A solution has the following composition in mol per cent: ethane 0.25%, propane 25.00%, isobutane 18.5%,n-butane 56.0% and isopentane 0.25%. For a pressure of
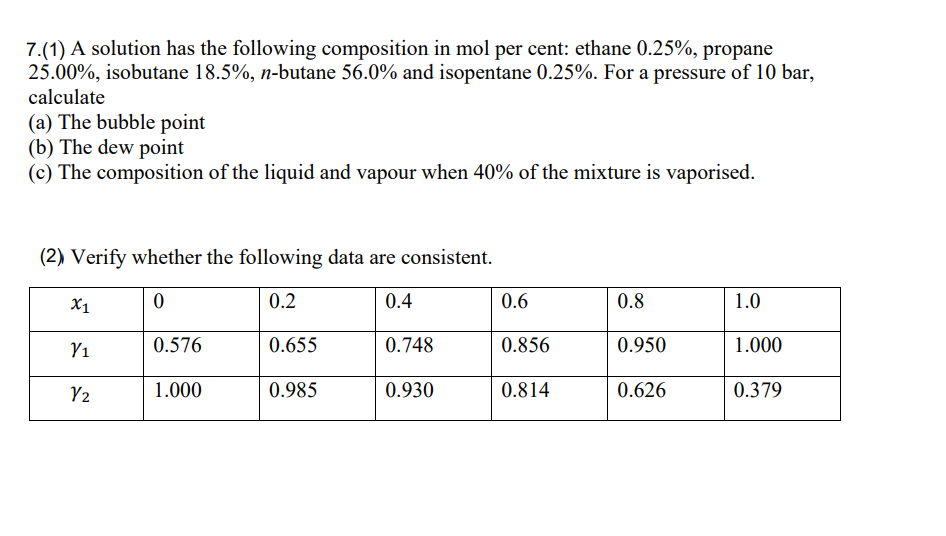
7.(1) A solution has the following composition in mol per cent: ethane 0.25%, propane 25.00%, isobutane 18.5%,n-butane 56.0% and isopentane 0.25%. For a pressure of 10 bar, calculate (a) The bubble point (b) The dew point (c) The composition of the liquid and vapour when 40% of the mixture is vaporised. (2) Verify whether the following data are consistent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


