A solution has the following composition, expressed as mol percentage: ethane, (0.25 %); propane, (25 %); isobutane,
Question:
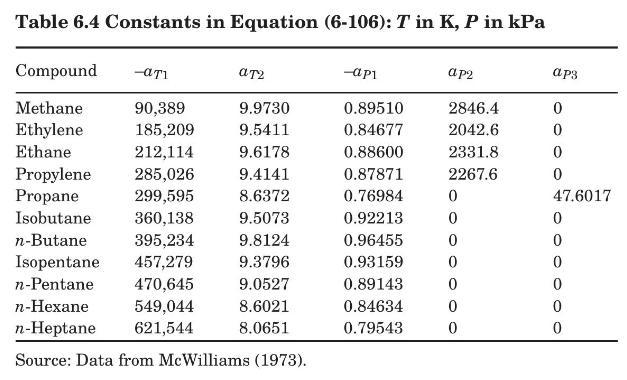
A solution has the following composition, expressed as mol percentage: ethane, \(0.25 \%\); propane, \(25 \%\); isobutane, \(18.5 \%\); \(n\)-butane, \(56 \%\); isopentane, \(0.25 \%\). In the following, the 3 pressure is 10 bars. Use equation (6-106) and Table 6.4 to calculate equilibrium distribution coefficients.
Table 6.4:-
Equation 6-106:-
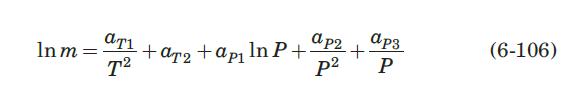
(a) Calculate the bubble point.
(b) Calculate the dew point.
(c) Forty mol\% of the feed is flash-vaporized. Calculate the temperature and composition of the products.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





