Question: 8. (28 pts.) Consider CIF3 molecule by taking only one p orbital on each fluorine atom with -symmetry, and valence PxxP5 and Pr2, orbite!s on
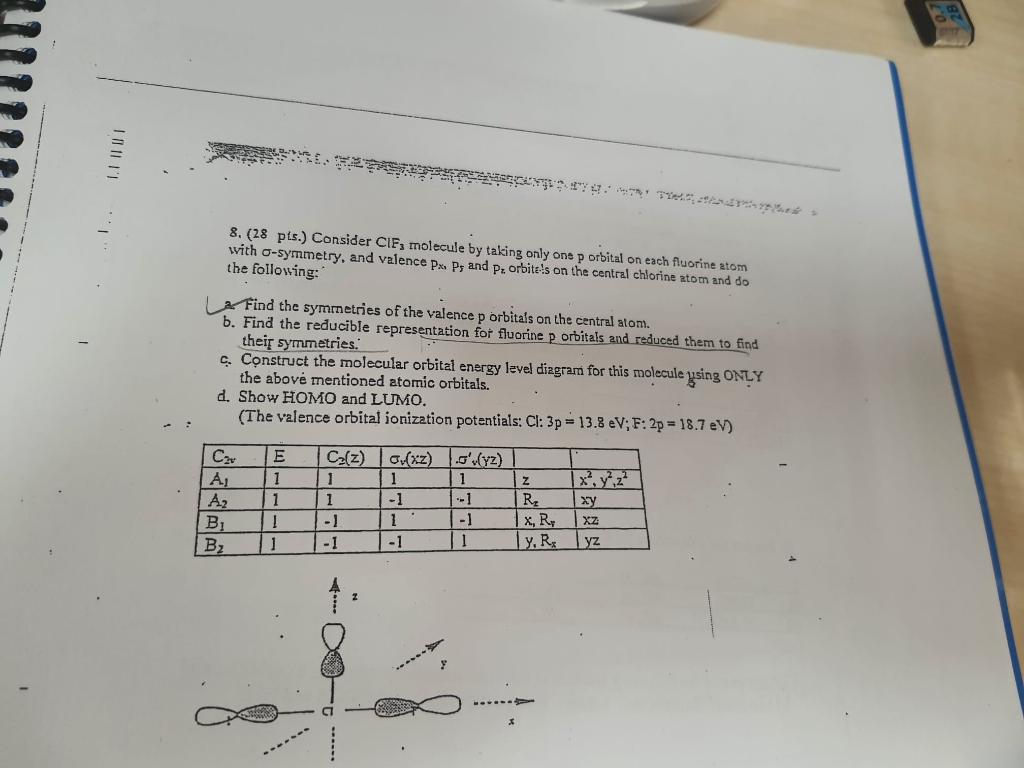
8. (28 pts.) Consider CIF3 molecule by taking only one p orbital on each fluorine atom with -symmetry, and valence PxxP5 and Pr2, orbite!s on the central chlorine atom and do the following: 2. Find the symmetries of the valence p orbitals on the central atom. b. Find the reducible representation for fluorine p orbitals and reduced them to find their symmetries: c. Construct the molecular orbital energy level diagram for this molecule using ONLY the above mentioned atomic orbitals. d. Shoty EOMO and LUMO. (The valence orbital jonization potentials: Cl: 3p=13.8eV;F:2p=18.7eV )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


