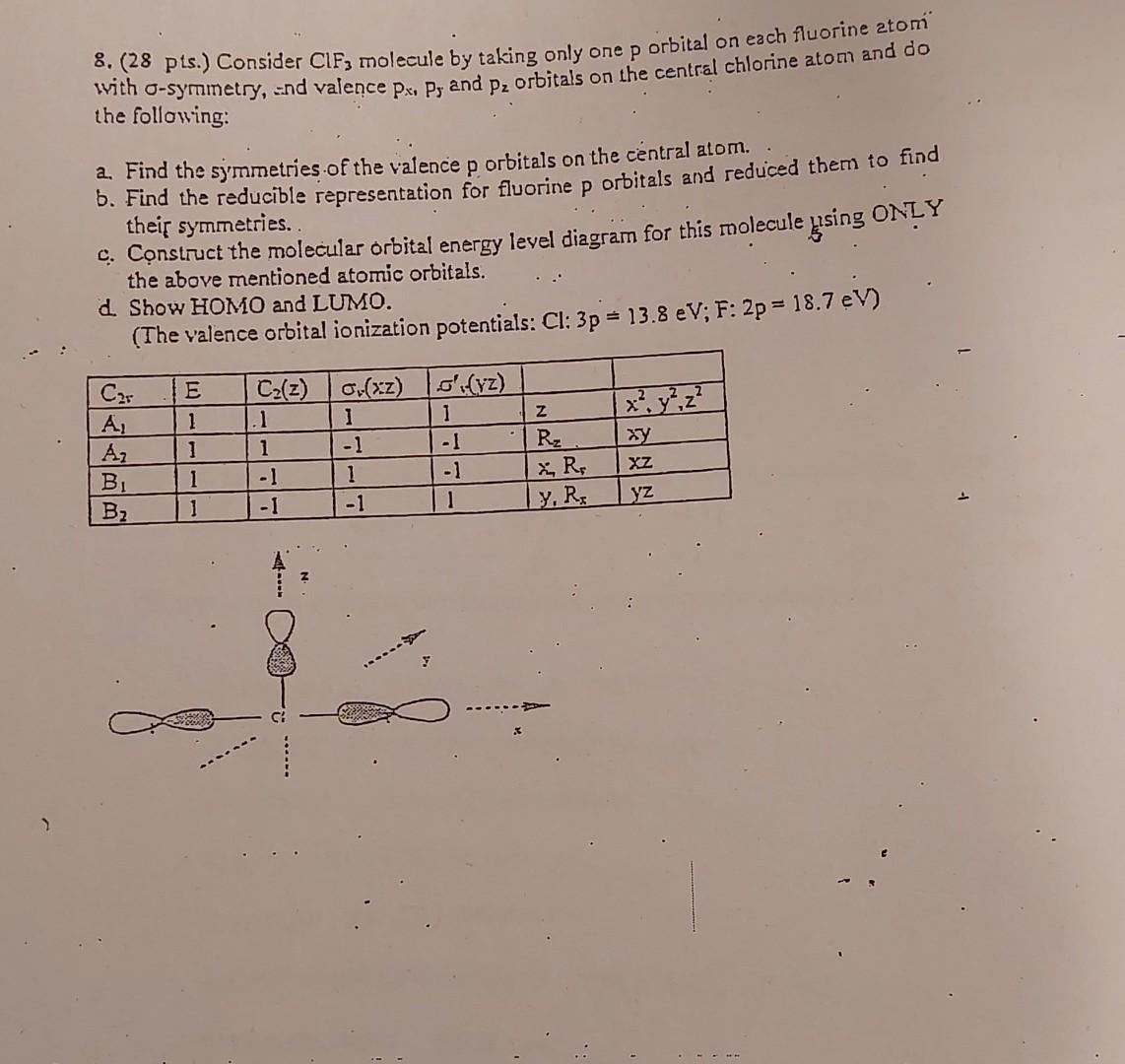
Question: How can we comment and solve the Wash Model? 8. ( 28 pts.) Consider ClF3 molecule by taking only one p orbital on each fluorine
How can we comment and solve the Wash Model?
8. ( 28 pts.) Consider ClF3 molecule by taking only one p orbital on each fluorine atomi with -symmetry, = nd valence px,p5 and pz orbitals on the central chlorine atom and do the following: a. Find the symmetries of the valence p orbitals on the central atom. . b. Find the reducible representation for fluorine p orbitals and reduced them to find their symmetries. c. Construct the molecular orbital energy level diagram for this molecule ssingONIY the above mentioned atomic orbitals. d. Show HOMO and LUMO. (The valence orbital ionization potentials: Cl:3p=13.8eV;F:2p=18.7eV )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


