Question: A solution is made by mixing 30.0 mL30.0 mL of ethanol, C2H60,C2H60, and 70.0 mL70.0 mL of water. Assuming ideal behavior, what is the
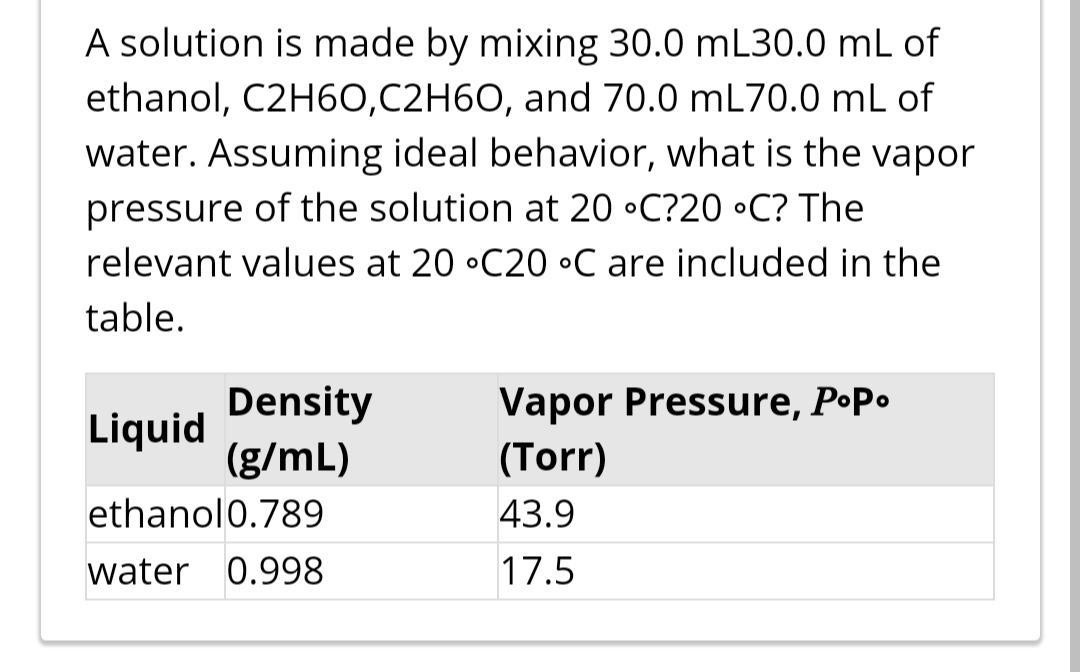
A solution is made by mixing 30.0 mL30.0 mL of ethanol, C2H60,C2H60, and 70.0 mL70.0 mL of water. Assuming ideal behavior, what is the vapor pressure of the solution at 20 C?20 C? The relevant values at 20 C20 C are included in the table. Density (g/mL) Vapor Pressure, PP. Liquid (Torr) ethanol0.789 43.9 water 0.998 17.5
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


