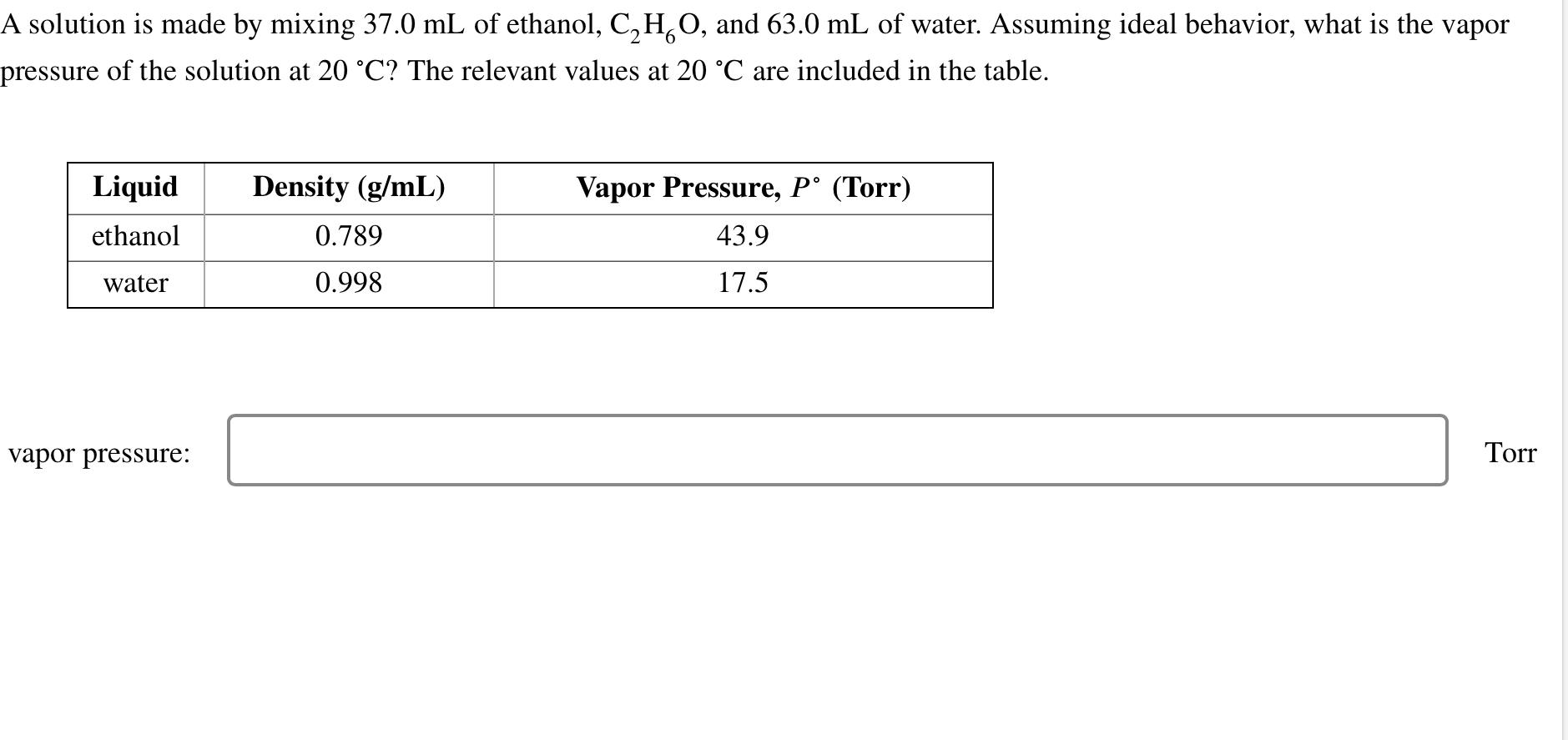
Question: A solution is made by mixing 3 7 . 0 m L of ethanol, C 2 H 6 O , and 6 3 . 0
A solution is made by mixing of ethanol, and of water. Assuming ideal behavior, what is the vapor pressure of the solution at The relevant values at are included in the table.
tableLiquidDensity Vapor Pressure, Torrethanolwater
vapor press
Torr
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


