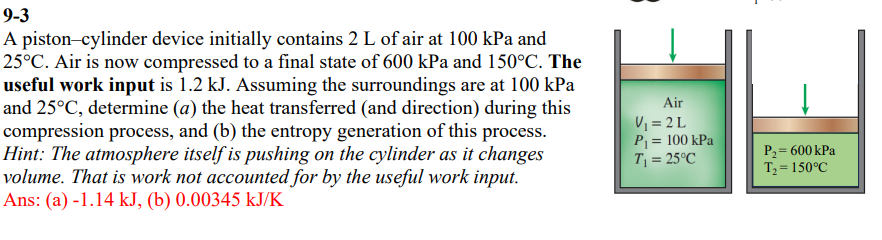
Question: ( 9 - 3 ) A piston - cylinder device initially contains 2 L of air at 1 0 0 kPa and
A pistoncylinder device initially contains L of air at kPa and circmathrmC Air is now compressed to a final state of kPa and circmathrmC The useful work input is kJ Assuming the surroundings are at kPa and circmathrmC determine a the heat transferred and direction during this compression process, and b the entropy generation of this process. Hint: The atmosphere itself is pushing on the cylinder as it changes volume. That is work not accounted for by the useful work input. Ans: a kJ bmathrm~kJmathrmK
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


