Question: (9) Construct a Born-Haber-cycle by adding the right processes and energies (1) to the diagram below and use it to calculate the enthalpy of formation
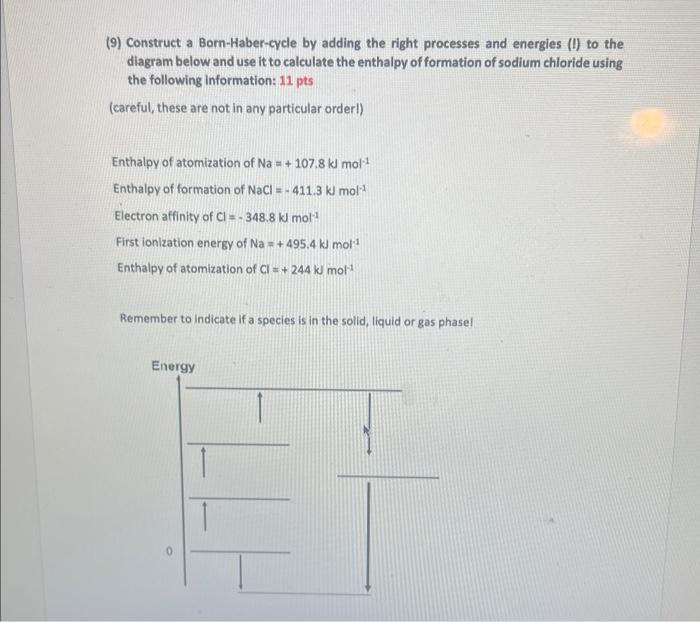
(9) Construct a Born-Haber-cycle by adding the right processes and energies (1) to the diagram below and use it to calculate the enthalpy of formation of sodium chloride using the following information: 11pts (careful, these are not in any particular orderl) Enthalpy of atomization of Na=+107.8kJmol1 Enthalpy of formation of NaCl=411.3kJmol1 Electron affinity of Cl=348.8klmol1 First ionization energy of Na=+495.4kmol1 Enthalpy of atomization of Cl=+244kmol3 Remember to Indicate if a species is in the solid, liquid or gas phasel
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


