Question: 9) Titration Curve Practice. (2.0 pt) Review your class notes on the titration of acetic acid (CH3 COOH ). Another way to construct titration curves
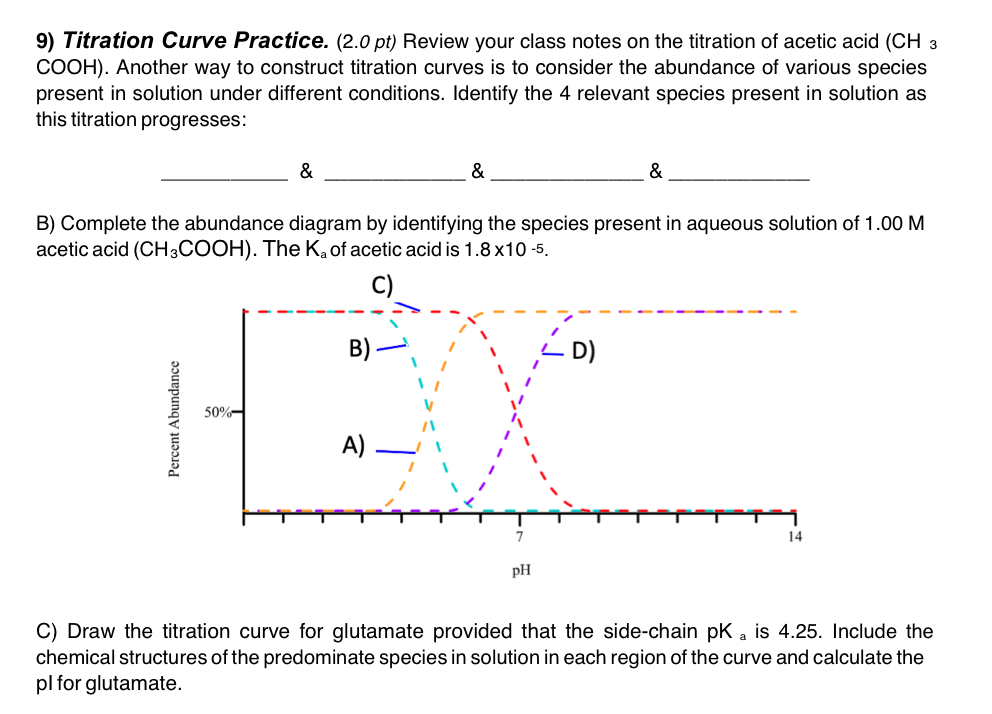
9) Titration Curve Practice. (2.0 pt) Review your class notes on the titration of acetic acid (CH3 COOH ). Another way to construct titration curves is to consider the abundance of various species present in solution under different conditions. Identify the 4 relevant species present in solution as this titration progresses: \& \& & B) Complete the abundance diagram by identifying the species present in aqueous solution of 1.00M acetic acid (CH3COOH). The Ka of acetic acid is 1.8105. C) Draw the titration curve for glutamate provided that the side-chain pKa is 4.25. Include the chemical structures of the predominate species in solution in each region of the curve and calculate the pl for glutamate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


