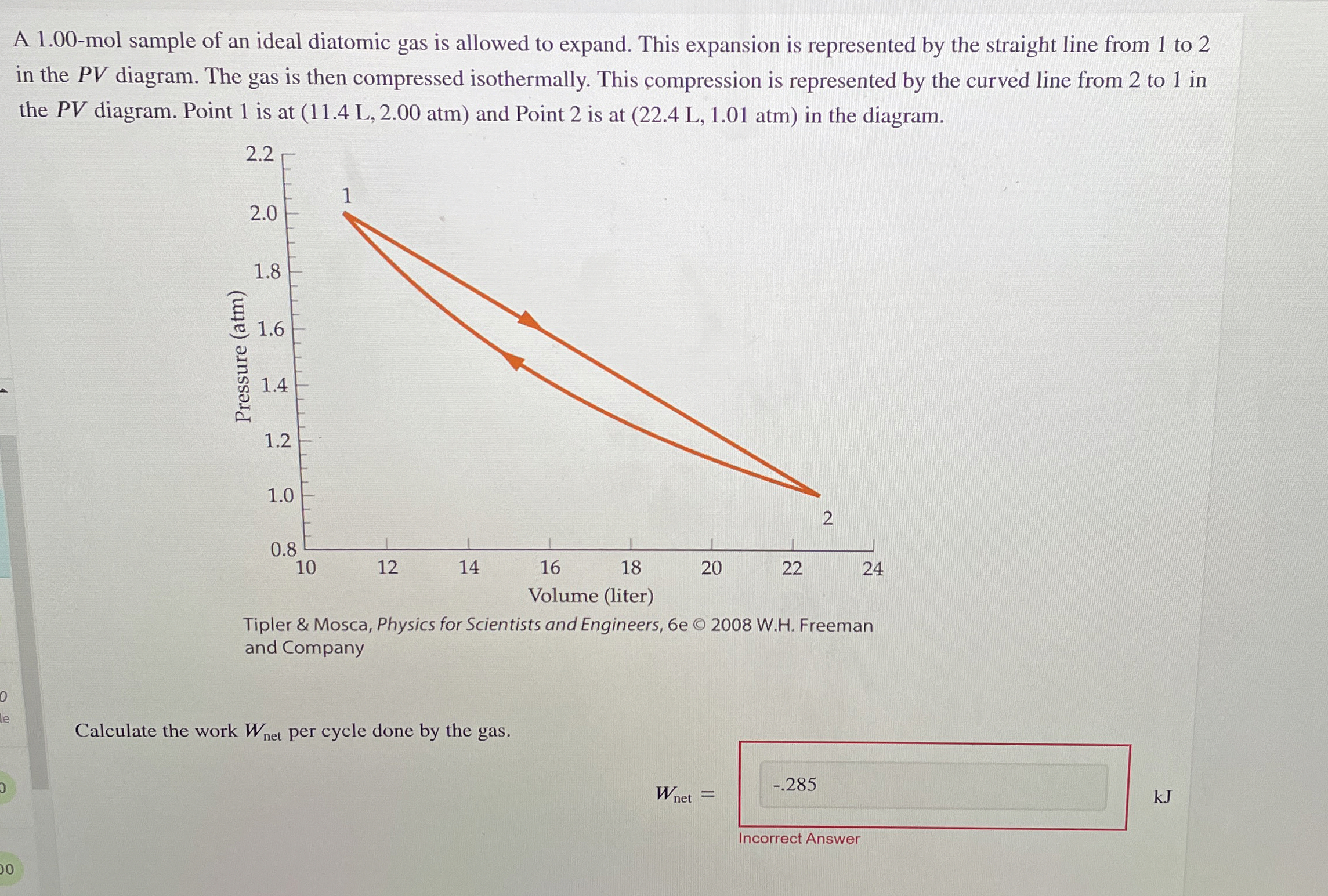
Question: A 1 . 0 0 - mol sample of an ideal diatomic gas is allowed to expand. This expansion is represented by the straight line
A mol sample of an ideal diatomic gas is allowed to expand. This expansion is represented by the straight line from to in the diagram. The gas is then compressed isothermally. This compression is represented by the curved line from to in the diagram. Point is at atm and Point is at atm in the diagram.
and Company
Calculate the work per cycle done by the gas.
kJ
Incorrect Answer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


