Question: A = 1 B = 9 X = 0 Y = 5 Z = 3 (Use A,B,X,Y, and Z values from your student number as
A = 1 B = 9
X = 0 Y = 5 Z = 3
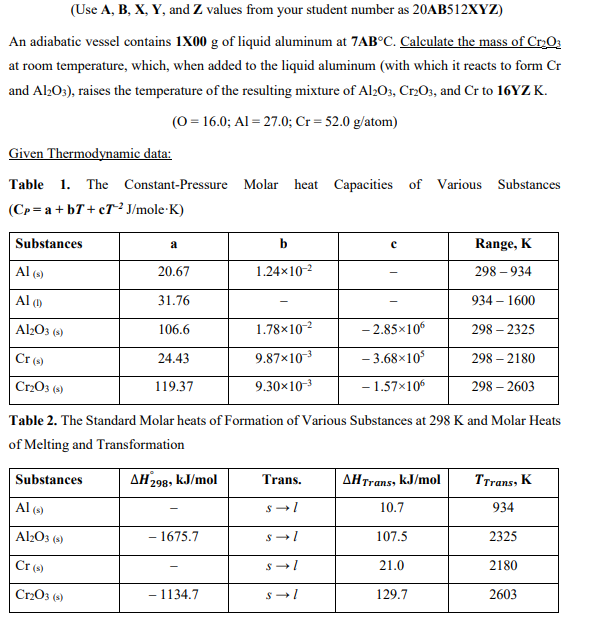
(Use A,B,X,Y, and Z values from your student number as 20AB512XZ ) An adiabatic vessel contains 1X00g of liquid aluminum at 7ABC. Calculate the mass of Cr2O3 at room temperature, which, when added to the liquid aluminum (with which it reacts to form Cr and Al2O3 ), raises the temperature of the resulting mixture of Al2O3,Cr2O3, and Cr to 16YZ K. (O=16.0;Al=27.0;Cr=52.0g/atom) Given Thermodynamic data: Table 1. The Constant-Pressure Molar heat Capacities of Various Substances (CP=a+bT+cT2J/moleK) Table 2. The Standard Molar heats of Formation of Various Substances at 298K and Molar Heats of Melting and Transformation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


