Question: A 103 molal aqueous solution of a chromium complex having same number of ammonia molecule and Cl ions freezes at -0.00558C. If all the
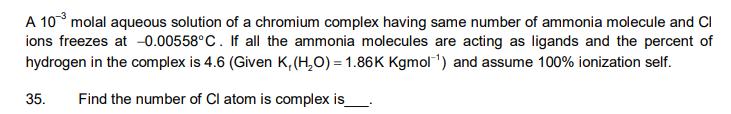
![]()
A 103 molal aqueous solution of a chromium complex having same number of ammonia molecule and Cl ions freezes at -0.00558C. If all the ammonia molecules are acting as ligands and the percent of hydrogen in the complex is 4.6 (Given K, (HO) = 1.86K Kgmol ) and assume 100% ionization self. 35. Find the number of Cl atom is complex is 36. The value of Vant Hoff factor is
Step by Step Solution
★★★★★
3.40 Rating (153 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The detailed ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


