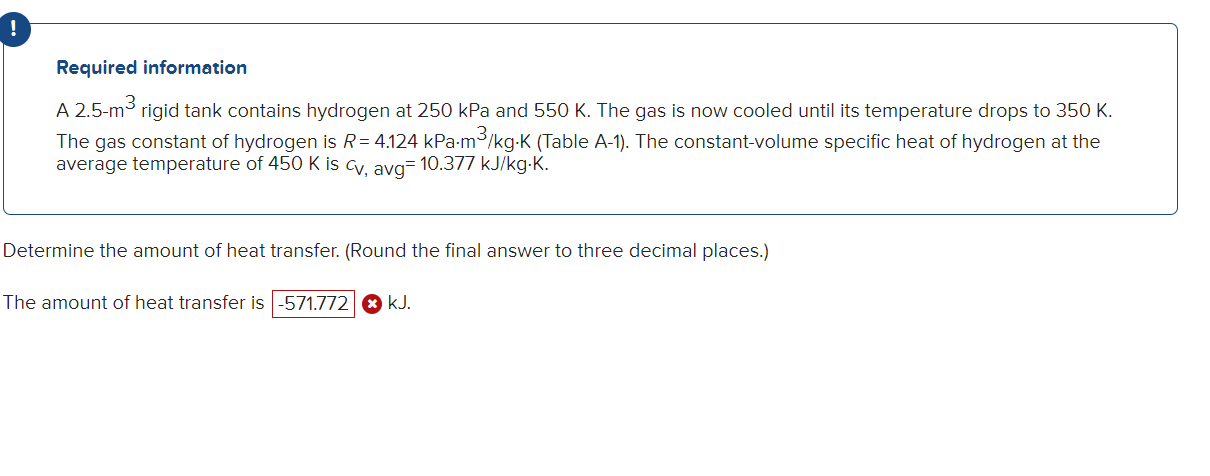
Question: A 2 . 5 - m 3 rigid tank contains hydrogen at 2 5 0 kPa and 5 5 0 K . The gas is
A m rigid tank contains hydrogen at kPa and K The gas is now cooled until its temperature drops to K The gas constant of hydrogen is R kPamkgK Table A The constantvolume specific heat of hydrogen at the average temperature of K is cv avg kJkgK
Determine the amount of heat transfer. Round the final answer to three decimal places.
The amount of heat transfer is Numeric ResponseEdit Unavailable. incorrect.kJRequired information
A rigid tank contains hydrogen at kPa and K The gas is now cooled until its temperature drops to K
The gas constant of hydrogen is kPaTable A The constantvolume specific heat of hydrogen at the
average temperature of K is avg
Determine the amount of heat transfer. Round the final answer to three decimal places.
The amount of heat transfer is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


