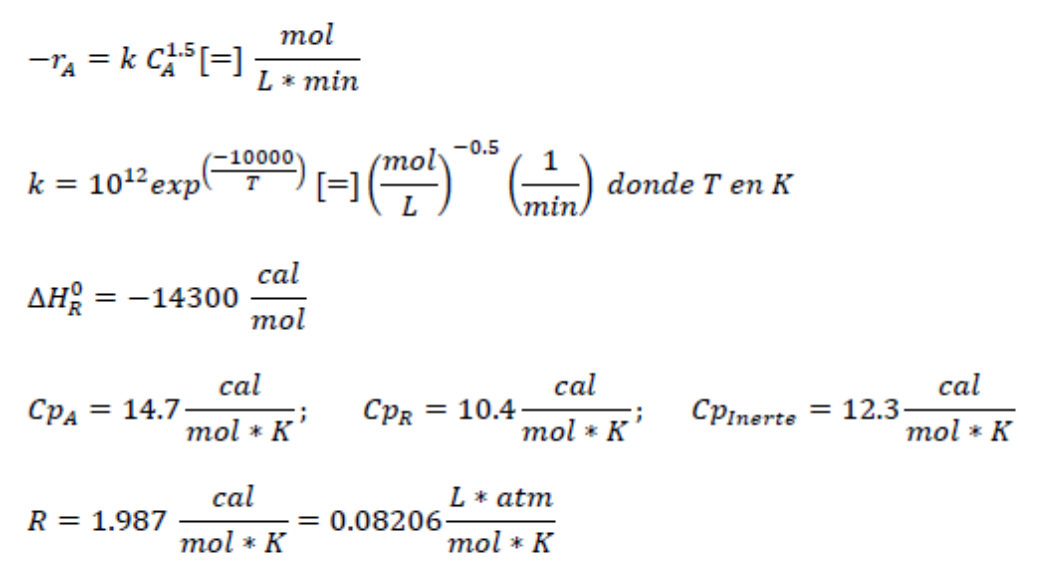
Question: A - > 2 R The previous reaction is carried out in the gas phase within a CSTR reactor. The feed stream consists of 2
AR
The previous reaction is carried out in the gas phase within a CSTR reactor. The feed stream consists of Lmin of a mixture at deg C and atm whose composition is mol of A and mol of an inert gas, the conversion is desired to be
Additional Data:Consider that the values of the heat capacities are constant over the temperature range.
exp whereT
;;
FORM:
Consider that the reactor must operate at a constant temperature of deg C Calculate the value of k at the operating temperature of the reactor.
Determine the value of CA in molmConcentration of A
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


