Question: A 3.4842 g sample of a solid mixture containing benzoic acid (C,H,COOH, 122.123 g mol') was dissolved and titrated with strong base to a
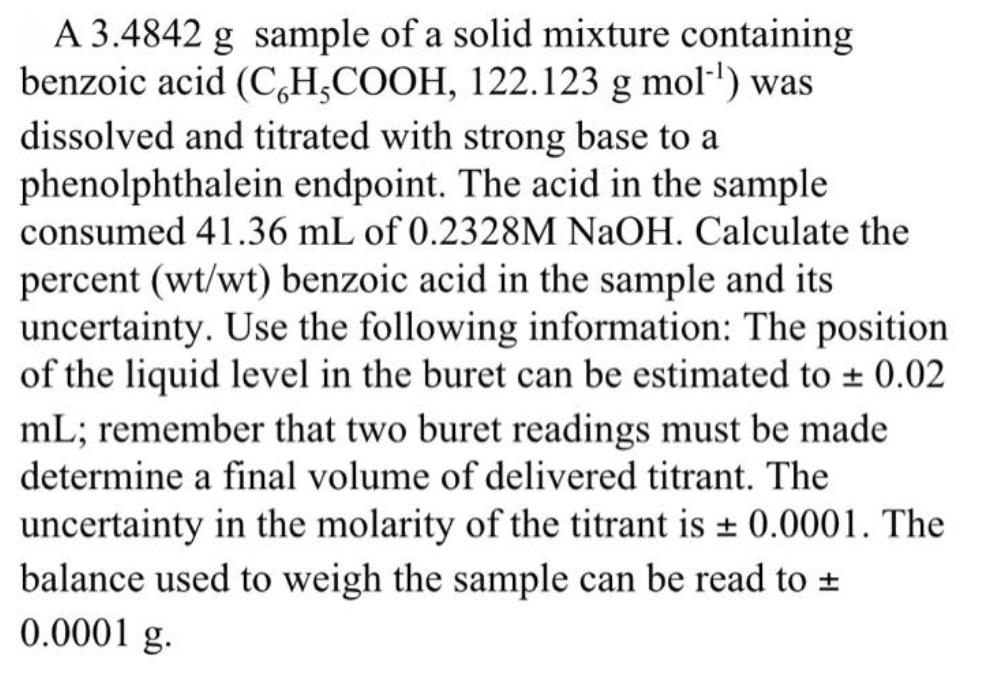
A 3.4842 g sample of a solid mixture containing benzoic acid (C,H,COOH, 122.123 g mol') was dissolved and titrated with strong base to a phenolphthalein endpoint. The acid in the sample consumed 41.36 mL of 0.2328M NaOH. Calculate the percent (wt/wt) benzoic acid in the sample and its uncertainty. Use the following information: The position of the liquid level in the buret can be estimated to 0.02 mL; remember that two buret readings must be made determine a final volume of delivered titrant. The uncertainty in the molarity of the titrant is + 0.0001. The balance used to weigh the sample can be read to + 0.0001 g.
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it
Volume of NaOH Final volume initial volume Given that the volume measurement has uncertainity of 002 ... View full answer

Get step-by-step solutions from verified subject matter experts


