Question: a) A mass spectrometric analysis revealed that copper has two naturally occurring isotopes. The data is summarized below: Isotope 1: 62.9296amu Isotope 2: 64.9278amu If
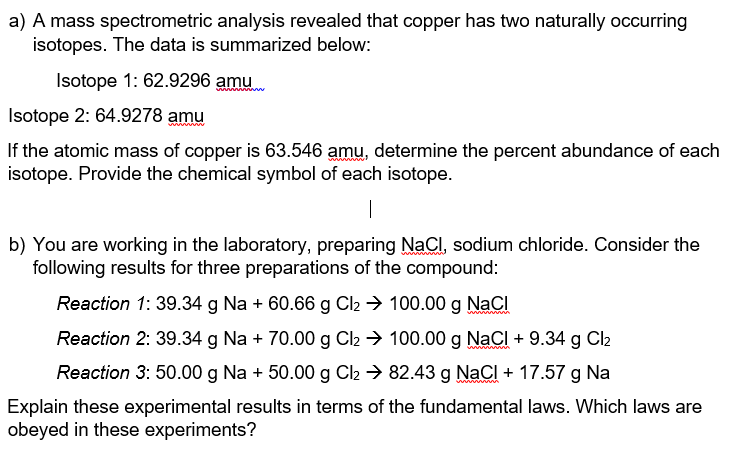
a) A mass spectrometric analysis revealed that copper has two naturally occurring isotopes. The data is summarized below: Isotope 1: 62.9296amu Isotope 2: 64.9278amu If the atomic mass of copper is 63.546amu, determine the percent abundance of each isotope. Provide the chemical symbol of each isotope. b) You are working in the laboratory, preparing NaCl, sodium chloride. Consider the following results for three preparations of the compound: Reaction 1: 39.34gNa+60.66gCl2100.00gNaCl Reaction 2: 39.34gNa+70.00gCl2100.00gNaCl+9.34gCl2 Reaction 3: 50.00gNa+50.00gCl282.43gNaCl+17.57gNa Explain these experimental results in terms of the fundamental laws. Which laws are obeyed in these experiments
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


