Question: Please help and be clear thanks Question 26 Treating 1,2-cyclohexanediol with concentrated sulfuric acid yields a product with molecular formula C6H100. An IR spectrum of
Please help and be clear thanks 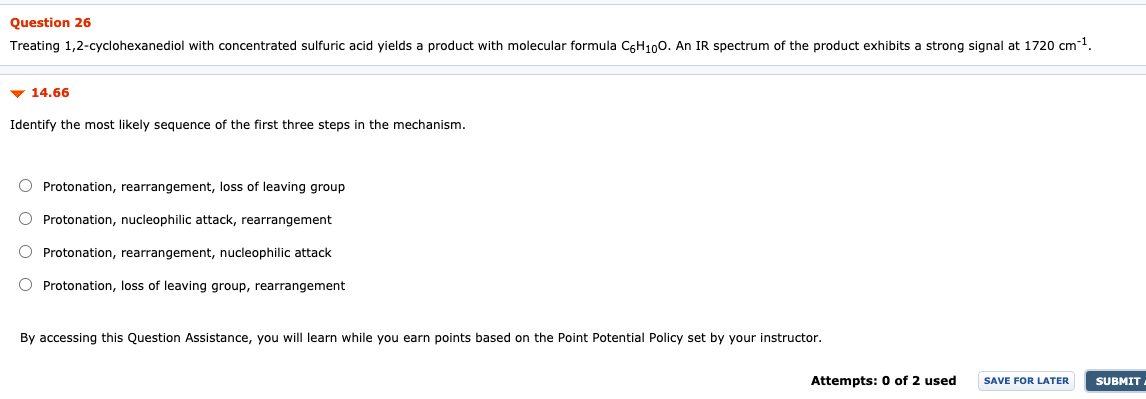
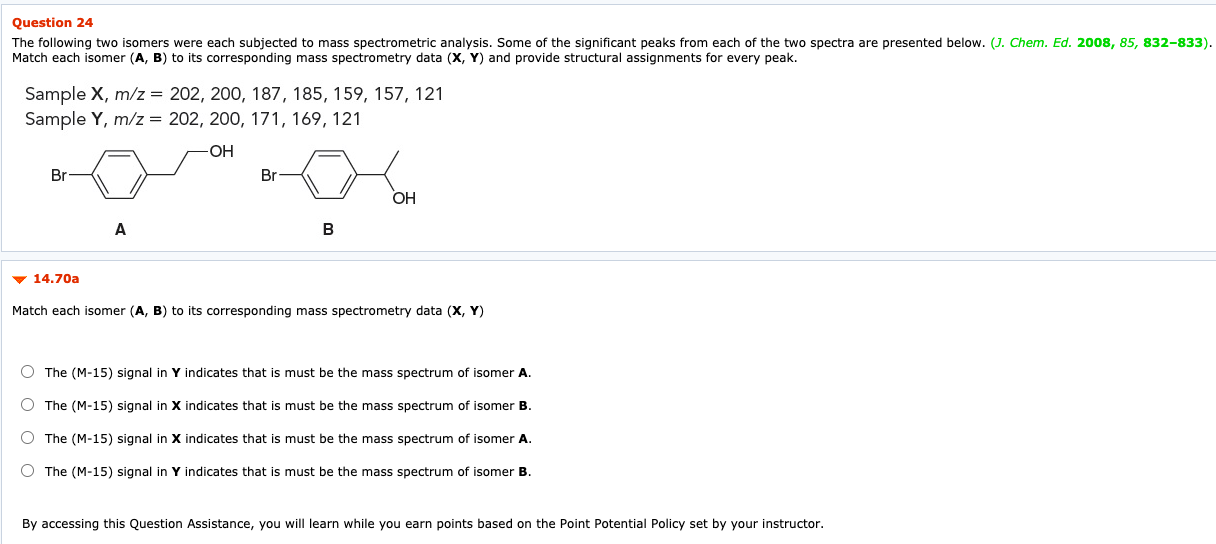
Question 26 Treating 1,2-cyclohexanediol with concentrated sulfuric acid yields a product with molecular formula C6H100. An IR spectrum of the product exhibits a strong signal at 1720 cm-1 14.66 Identify the most likely sequence of the first three steps in the mechanism. O Protonation, rearrangement, loss of leaving group O Protonation, nucleophilic attack, rearrangement O Protonation, rearrangement, nucleophilic attack O Protonation, loss of leaving group, rearrangement By accessing this Question Assistance, you will learn while you earn points based on the Point Potential Policy set by your instructor. Attempts: 0 of 2 used SAVE FOR LATER SUBMIT Question 24 The following two isomers were each subjected to mass spectrometric analysis. Some of the significant peaks from each of the two spectra are presented below. (J. Chem. Ed. 2008, 85, 832-833). Match each isomer (A, B) to its corresponding mass spectrometry data (X, Y) and provide structural assignments for every peak. Sample X, m/z = 202, 200, 187, 185, 159, 157, 121 Sample Y, m/z = 202, 200, 171, 169, 121 Br Br . A B 14.70a Match each isomer (A, B) to its corresponding mass spectrometry data (X, Y) O The (M-15) signal in Y indicates that is must be the mass spectrum of isomer A. The (M-15) signal in X indicates that is must be the mass spectrum of isomer B. The (M-15) signal in X indicates that is must be the mass spectrum of isomer A. The (M-15) signal in Y indicates that is must be the mass spectrum of isomer B. By accessing this Question Assistance, you will learn while you earn points based on the Point Potential Policy set by your instructor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


