Question: a) A second order gas reaction A+ B 2R +S proceeds in a constant pressure batch reactor where its volume increases 25% in 6
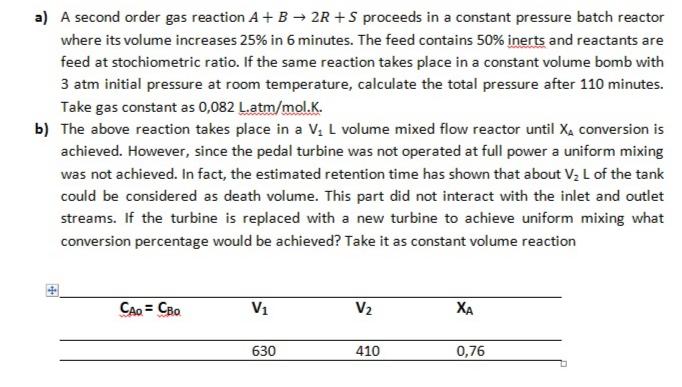
a) A second order gas reaction A+ B 2R +S proceeds in a constant pressure batch reactor where its volume increases 25% in 6 minutes. The feed contains 50% inerts and reactants are feed at stochiometric ratio. If the same reaction takes place in a constant volume bomb with 3 atm initial pressure at room temperature, calculate the total pressure after 110 minutes. Take gas constant as 0,082 L.atm/mol.K. b) The above reaction takes place in a V, L volume mixed flow reactor until Xa conversion is achieved. However, since the pedal turbine was not operated at full power a uniform mixing was not achieved. In fact, the estimated retention time has shown that about V2 L of the tank could be considered as death volume. This part did not interact with the inlet and outlet streams. If the turbine is replaced with a new turbine to achieve uniform mixing what conversion percentage would be achieved? Take it as constant volume reaction CAo = CBo V1 V2 XA 630 410 0,76
Step by Step Solution
3.48 Rating (138 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


