Question: a) At steady state condition determine the diffusion rate of oxygen (A) through a non- diffusing gases first nitrogen (B) and then carbon dioxide (C),
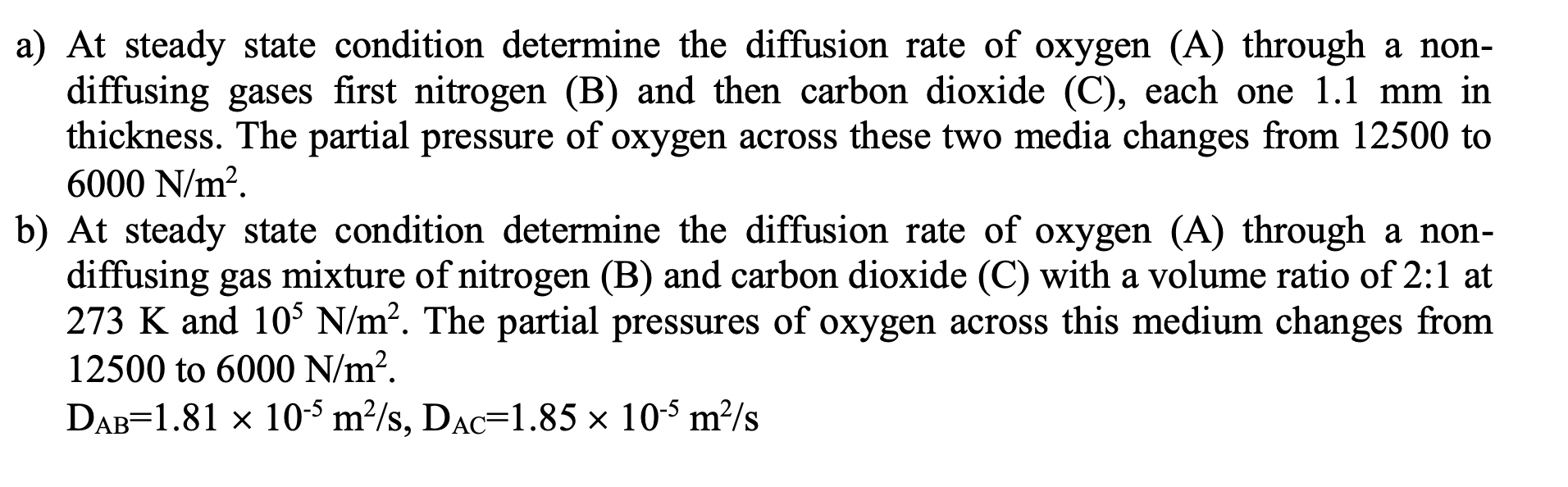
a) At steady state condition determine the diffusion rate of oxygen (A) through a non- diffusing gases first nitrogen (B) and then carbon dioxide (C), each one 1.1 mm in thickness. The partial pressure of oxygen across these two media changes from 12500 to 6000 N/m2. b) At steady state condition determine the diffusion rate of oxygen (A) through a non- diffusing gas mixture of nitrogen (B) and carbon dioxide (C) with a volume ratio of 2:1 at 273 K and 10% N/m. The partial pressures of oxygen across this medium changes from 12500 to 6000 N/m. DAB=1.81 x 10-4 m/s, Dac=1.85 x 10-5 m/s a
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


