Question: . A . B C pressure (atm) E F H temperature (K) A OB OC D If a sample of pure X is to be
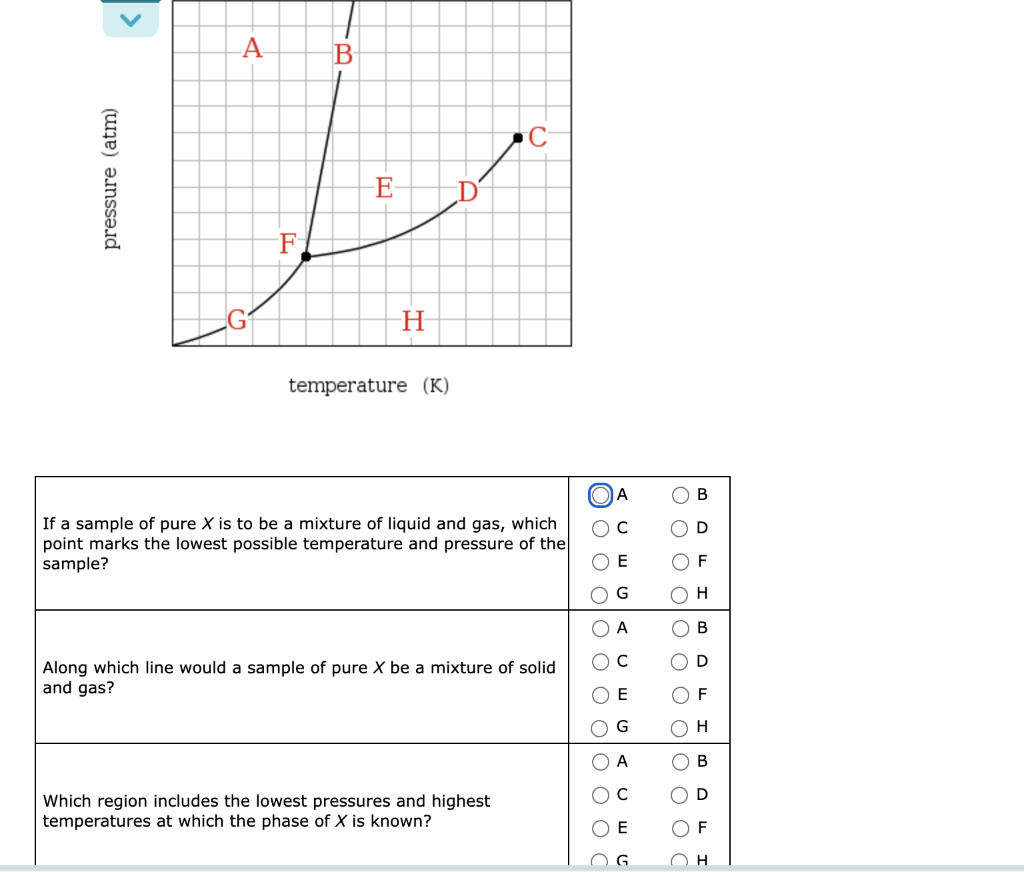
. A . B C pressure (atm) E F H temperature (K) A OB OC D If a sample of pure X is to be a mixture of liquid and gas, which point marks the lowest possible temperature and pressure of the sample? OF Od 0 G O O O O O O O H B OD Along which line would a sample of pure X be a mixture of solid and gas? OE OF G OH A D Which region includes the lowest pressures and highest temperatures at which the phase of X is known? O O Od E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


