Question: A. B. What is the dominant intermolecular force in a pure sample of ethyl acetate? C. Order the following compounds from lowest (1) to highest
A.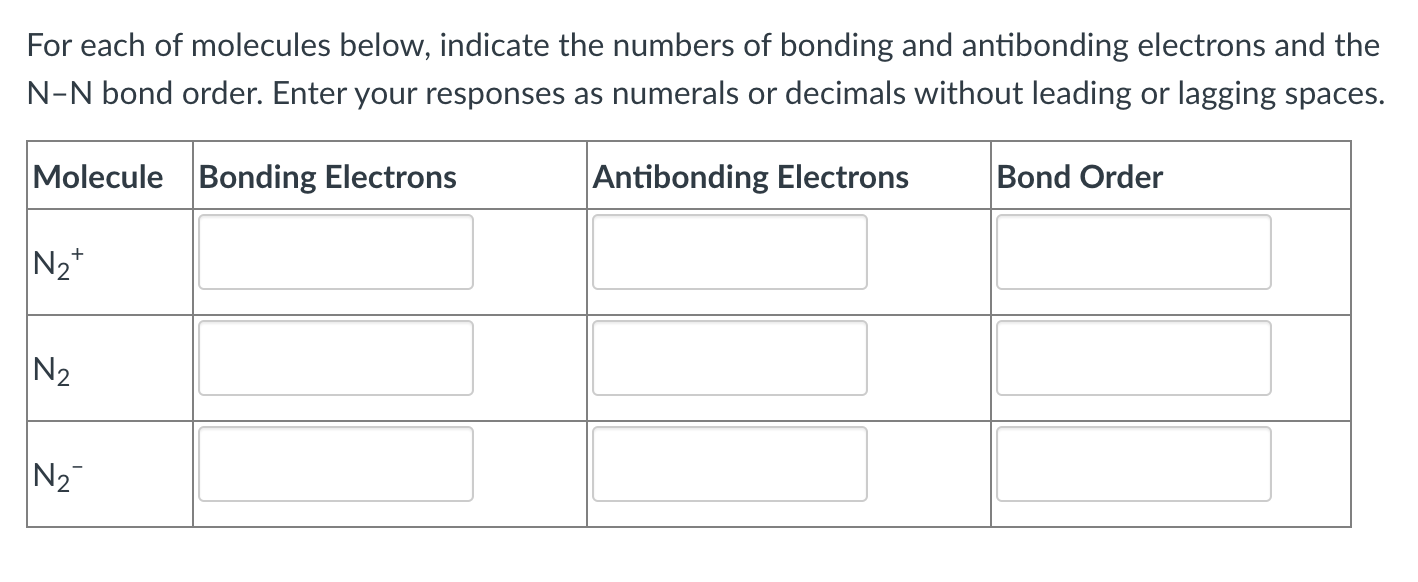 B. What is the dominant intermolecular force in a pure sample of ethyl acetate?
B. What is the dominant intermolecular force in a pure sample of ethyl acetate?
C. Order the following compounds from lowest (1) to highest (5) vapor pressure at 300 K.
A CH3OCH2CH2OCH3 B HOCH2CH2OCH2CH2OH C HOCH2CH2OH D HOCH2CH2OCH2CH2OCH2CH2OH E HOCH2CH2OCH3
D. Elemental nickel crystallizes in the face-centered cubic crystal structure; its molar mass is 58.69 g/mol. What is the total mass of atoms enclosed by a single unit cell in this structure? Enter your response in atomic mass units (amu) to the nearest 0.1 amu.
For each of molecules below, indicate the numbers of bonding and antibonding electrons and the N-N bond order. Enter your responses as numerals or decimals without leading or lagging spaces. Molecule Bonding Electrons Antibonding Electrons Bond Order N2+ N2 N2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


