Question: please help with post lab questions Post Lab Questions: Simple & Fractional Distillation ( 7 pts) In your lab report answer the following questions and
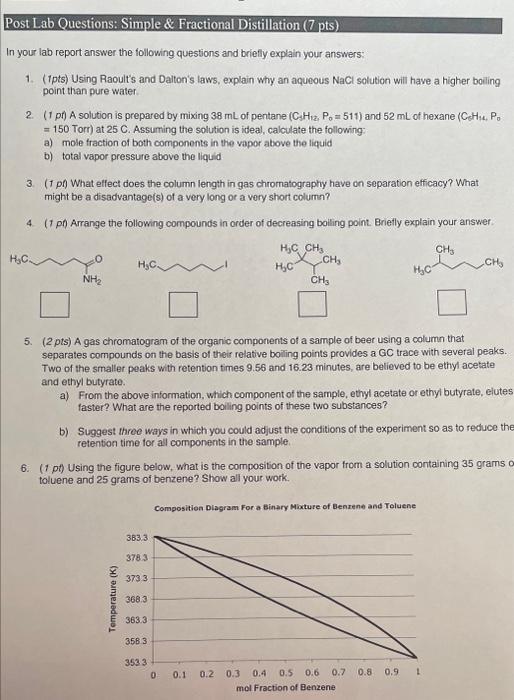
Post Lab Questions: Simple \& Fractional Distillation ( 7 pts) In your lab report answer the following questions and briefly explain your answers: 1. (1pts) Using Raoult's and Dalton's laws, explain why an aqueous NaCl solution will have a higher boiling point than pure water. 2. (1 pt) A solution is prepared by mixing 38mL of pentane (C3H1,P0=511) and 52mL of hexane (C-CH, H14. =150 Torr) at 25C. Assuming the solution is ideal, calculate the following: a) mole fraction of both components in the vapor above the liquid b) total vapor pressure above the liquid 3. (f p ) What elfect does the column length in gas chromatography have on separation efficacy? What might be a disadvantage(s) of a very long or a very short column? 4. (I pt) Arrange the following compounds in order of decreasing bolling point. Brietly explain your answer. 5. (2 pts) A gas chromatogram of the organic components of a sample of beer using a column that separates compounds on the basis of their relative boiling points provides a GC trace with several peaks. Two of the smaller peaks with retention times 9.56 and 16.23 minutes, are believed to be ethyl acetate and ethyl butyrate. a) From the above information, which component of the sample, ethyl acetate or ethyl butyrate, elutes faster? What are the reported boiling points of these two substances? b) Suggest three ways in which you could adjust the conditions of the experiment so as to reduce the retention time for al components in the sample. 6. ( 1 pt) Using the figure below, what is the composition of the vapor from a solution containing 35 grams 0 toluene and 25 grams of benzene? Show all your work. Compositien Dlagram For a Binary Moxture of Elenrend and Toluene
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


