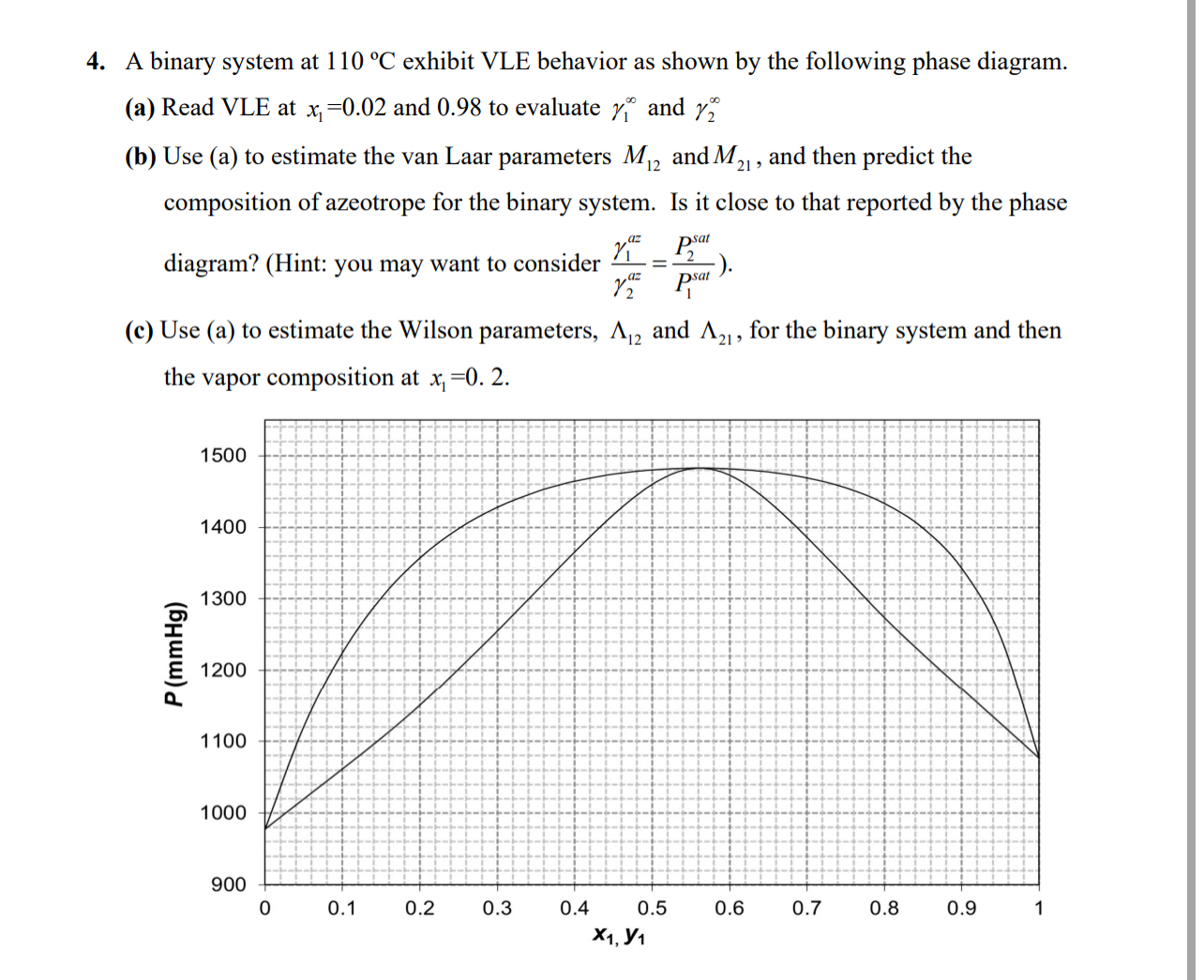
Question: A binary system at 1 1 0 C exhibit VLE behavior as shown by the following phase diagram. ( a ) Read VLE at x
A binary system at exhibit VLE behavior as shown by the following phase diagram.
a Read VLE at and to evaluate and
b Use a to estimate the van Laar parameters and and then predict the composition of azeotrope for the binary system. Is it close to that reported by the phase diagram? Hint: you may want to consider
c Use a to estimate the Wilson parameters, and for the binary system and then the vapor composition at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


