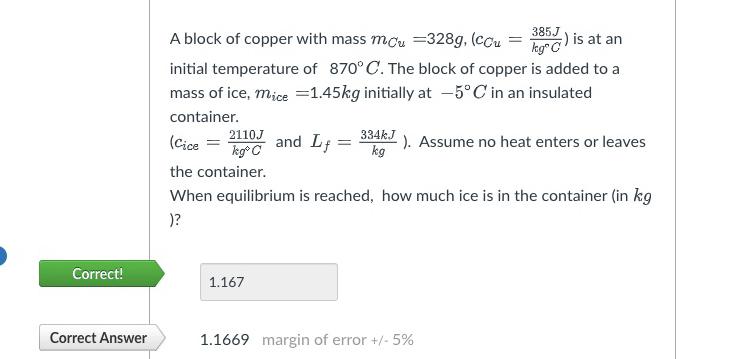
Question: A block of copper with mass ) = ( 3 8 5 ( J ) k k g C is at an initial temperature of
A block of copper with mass is at an initial temperature of The block of copper is added to a mass of ice, initially at in an insulated container.
and : Assume no heat enters or leaves the container.
When equilibrium is reached, how much ice is in the container in
Correct!
Correct Answer
margin of error
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


