Question: D. HANDWRITTEN THEN BOX THE FINAL ANSWERS DO NOT WRITE IN CURSIVE HEAT 1. A 45 grams of copper is heated from 35 .C to
D. HANDWRITTEN THEN BOX THE FINAL ANSWERS
DO NOT WRITE IN CURSIVE
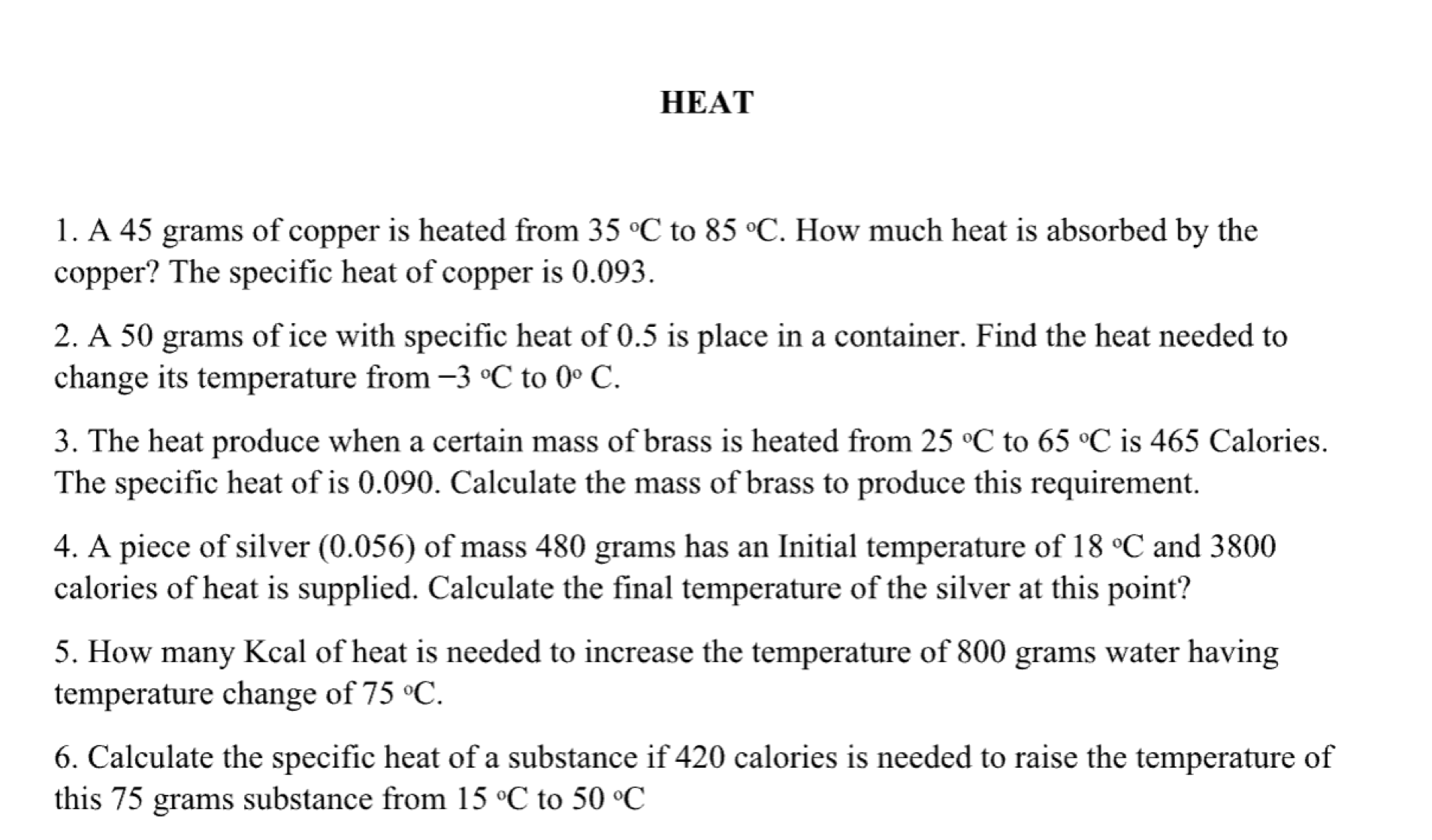
HEAT 1. A 45 grams of copper is heated from 35 .C to 85 .C. How much heat is absorbed by the copper? The specific heat of copper is 0.093. 2. A 50 grams of ice with specific heat of 0.5 is place in a container. Find the heat needed to change its temperature from -3 C to 0 C. 3. The heat produce when a certain mass of brass is heated from 25 .C to 65 .C is 465 Calories. The specific heat of is 0.090. Calculate the mass of brass to produce this requirement. 4. A piece of silver (0.056) of mass 480 grams has an Initial temperature of 18 .C and 3800 calories of heat is supplied. Calculate the final temperature of the silver at this point? 5. How many Kcal of heat is needed to increase the temperature of 800 grams water having temperature change of 75 .C. 6. Calculate the specific heat of a substance if 420 calories is needed to raise the temperature of this 75 grams substance from 15 C to 50 .C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


