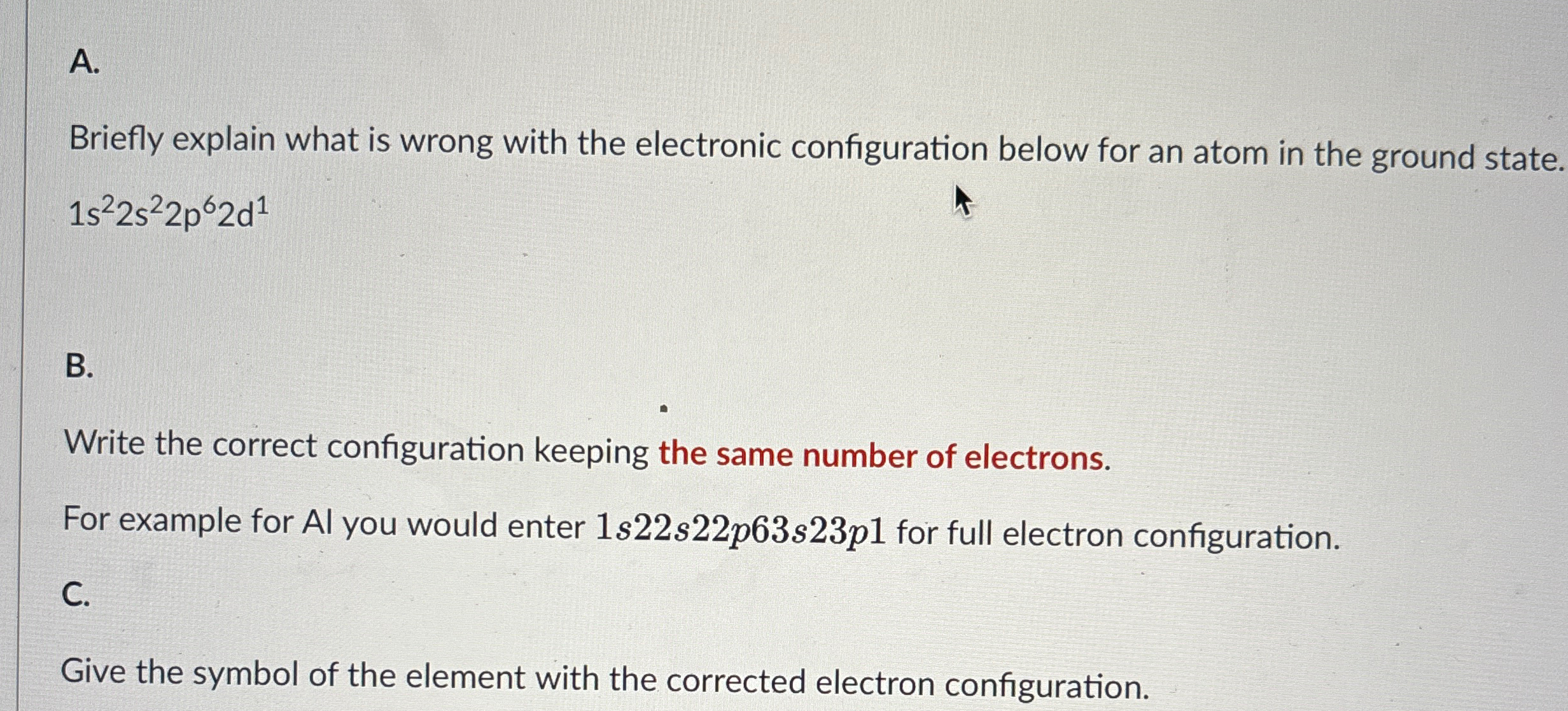
Question: A . Briefly explain what is wrong with the electronic configuration below for an atom in the ground state. 1 s 2 2 s 2
A Briefly explain what is wrong with the electronic configuration below for an atom in the ground state.
B
Write the correct configuration keeping the same number of electrons.
For example for Al you would enter for full electron configuration.
C
Give the symbol of the element with the corrected electron configuration.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


