Question: A buffer solution contains 0.383 M NH4Cl and 0.460 M NH3 (ammonia). Determine the pH change when 0.111 mol HNO, is added to 1.00 L
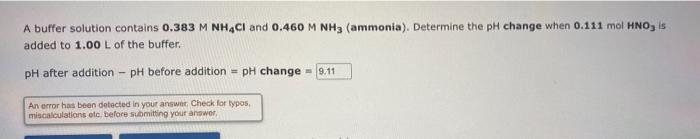
A buffer solution contains 0.383 M NH4Cl and 0.460 M NH3 (ammonia). Determine the pH change when 0.111 mol HNO, is added to 1.00 L of the buffer. pH after addition pH before addition = pH change = 9.11 An error has been detected in your answnt Check for typos, miscalculations etc. before submitting your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


