Question: A buffer solution contains 0.304 M C,H;NH,Br and 0.370 M C,H5NH, (aniline). Determine the pH change when 0.095 mol HBr is added to 1.00
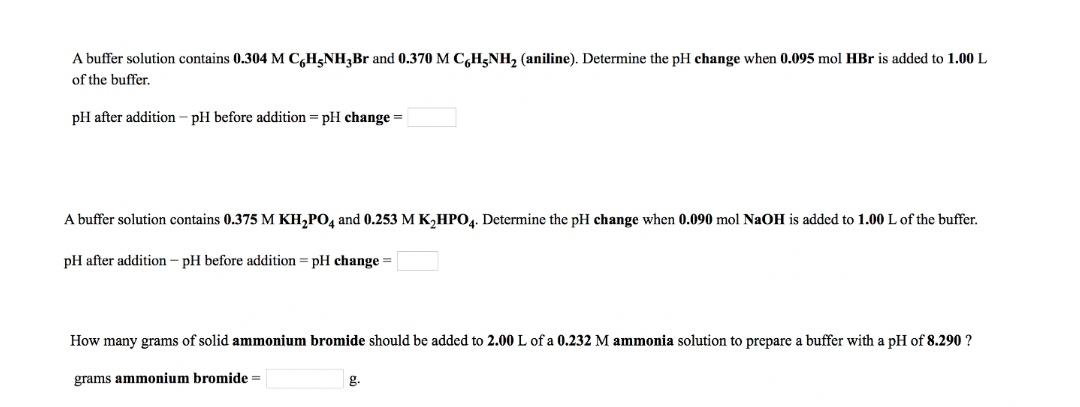
A buffer solution contains 0.304 M C,H;NH,Br and 0.370 M C,H5NH, (aniline). Determine the pH change when 0.095 mol HBr is added to 1.00 L of the buffer. pH after addition - pH before addition = pH change = A buffer solution contains 0.375 M KH,PO, and 0.253 M K,HPO4. Determine the pH change when 0.090 mol NaOH is added to 1.00 L of the buffer. pH after addition - pH before addition = pH change = How many grams of solid ammonium bromide should be added to 2.00 L of a 0.232 M ammonia solution to prepare a buffer with a pH of 8.290 ? grams ammonium bromide = g.
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


