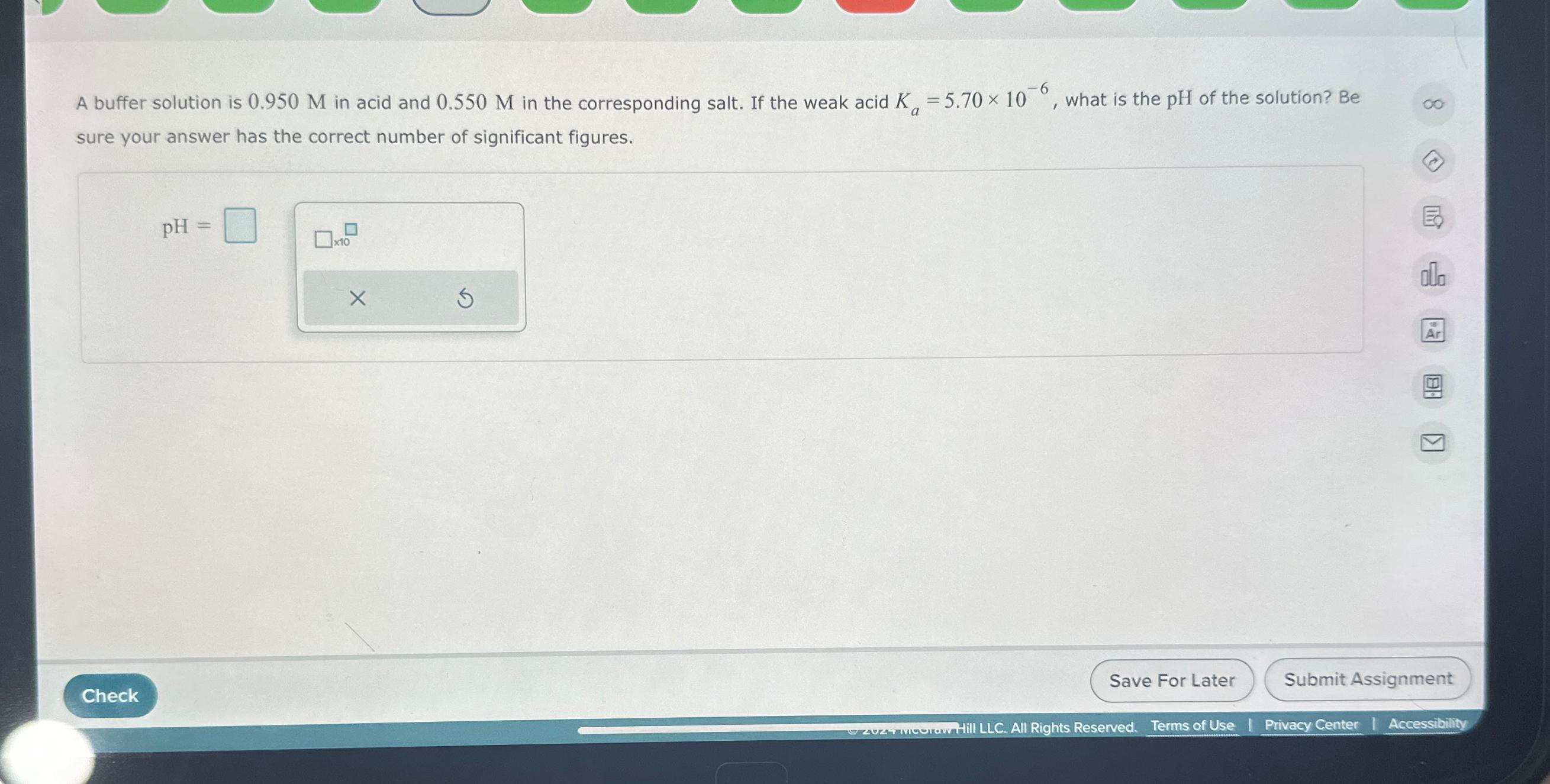
Question: A buffer solution is 0 . 9 5 0 M in acid and 0 . 5 5 0 M in the corresponding salt. If the
A buffer solution is in acid and in the corresponding salt. If the weak acid what is the pH of the solution? Be sure your answer has the correct number of significant figures.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


