Question: DATA: Labs MAKING THE BUFFER Target volume of conjugate base component: 53 mL Target volume of weak acid component: 47 mb Weak acid pk. 4.74
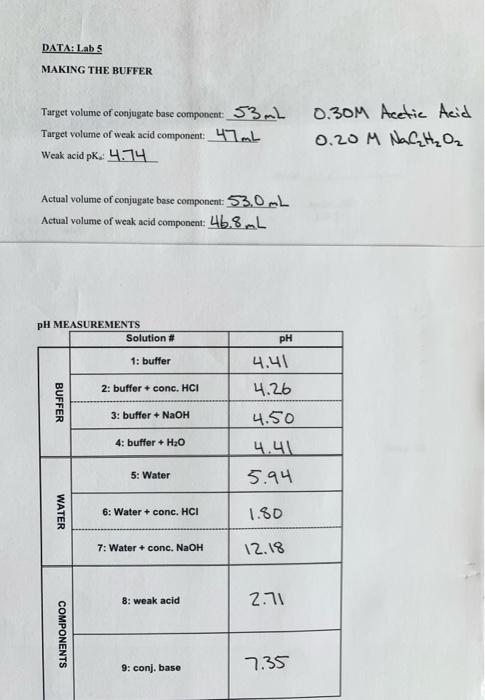


DATA: Labs MAKING THE BUFFER Target volume of conjugate base component: 53 mL Target volume of weak acid component: 47 mb Weak acid pk. 4.74 0.30M Acetic Acid 0.20 M NACHO Actual volume of conjugate base component: 53.0L Actual volume of wenk acid component: 46.8L pH MEASUREMENTS Solution # PH 1: buffer 4.41 4.26 BUFFER 2: buffer + conc. HCI 3: buffer + NaOH 4: buffer + H2O 4.50 4.41 5.94 5: Water WATER 6: Water + conc. HCI 1.80 7: Water + conc. NaOH 12.18 8: weak acid 2.71 COMPONENTS 9: conj, base 7.35 5. a) What was the pH change when you added, to your buffer: i) 1.00 mL of 0.5 M HCl(aq), making Solution 2? ii) 1.00 mL of 0.5 M NaOH(aq), making Solution 3? iii) 1.00 mL of pure H20 (1), making Solution 4? b) What was the pH change when you added to your water solution: i) 1.00 mL of 0.5 M HCl (aq), making Solution 6? ii) 1.00 mL of 0.5 M NaOH (aq), making Solution 72 Solution 1: Buffer (prepared above) Solution 2: -20 mL Buffer (prepared above) + 1.0 mL 0.5 M HCI Solution 3: -20 mL Buffer (prepared above) + 1.0 mL 0.5 M NaOH Solution 4: -20 mL Buffer (prepared above) + 1.0 mL distilled water Solution 5: Distilled Water Solution 6: -20 mL Distilled Water + 1.0 mL 0.5 M HCI ME Solution 7: ~20 mL Distilled Water + 1.0 mL 0.5 M NaOH Solution 8: Original Acetic Acid Solution babe Solution 9: Original Acetate Solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


