Question: A buffer solution with a pH of 4 . 0 is made by dissolving x g of potassium ethanoate ( C ? H 3 COO
A buffer solution with a pH of is made by dissolving of potassium ethanoate COO in a solution of ethanoic acid COO The final volume of the buffer solution is and the final concentration of the ethanoic acid is Calculate in for potassium ethanoate, the concentration of H ions where the answer is x moldm the concentration of CHCOO where the answer is x moldm Given
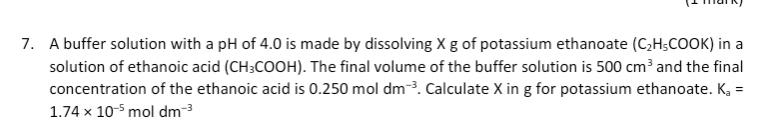
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


