Question: please answer everything!!! thank you so much Solve the following problems while observing the proper number of significant figures. Show your solutions. Atomic Weights: H
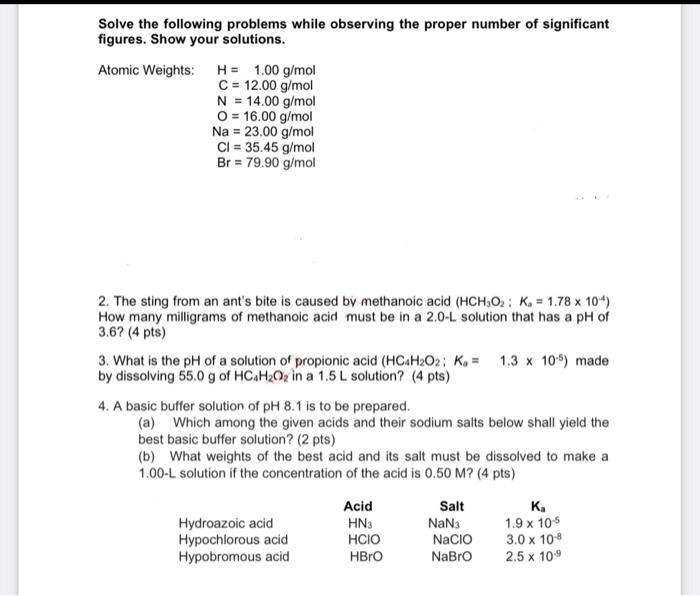
Solve the following problems while observing the proper number of significant figures. Show your solutions. Atomic Weights: H = 1.00 g/ mol C = 12.00 g/mol N = 14.00 g/mol = 16.00 g/mol Na = 23.00 g/mol Cl = 35.45 g/mol Br = 79.90 g/mol 2. The sting from an ant's bite is caused by methanoic acid (HCH,02: K = 1.78 x 104) How many milligrams of methanoic acid must be in a 2.0-L solution that has a pH of 3.6? (4 pts) 3. What is the pH of a solution of propionic acid (HC.H2O2; , = 1.3 x 109) made by dissolving 55.0 g of HC H20, in a 1.5 L solution? (4 pts) 4. A basic buffer solution of pH 8.1 is to be prepared. (a) Which among the given acids and their sodium salts below shall yield the best basic buffer solution? (2 pts) (b) What weights of the best acid and its salt must be dissolved to make a 1.00-L solution if the concentration of the acid is 0.50 M? (4 pts) Hydroazoic acid Hypochlorous acid Hypobromous acid Acid HN3 HCIO HBro Salt NaN3 Nacio NaBro K. 1.9 x 10-5 3.0 x 108 2.5 x 100
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


