Question: (a) Calculate the pH of a buffer solution made from 35.0 g of CH3COOH and 25.6 g of CH3COONa in 1.01 of solution. Given
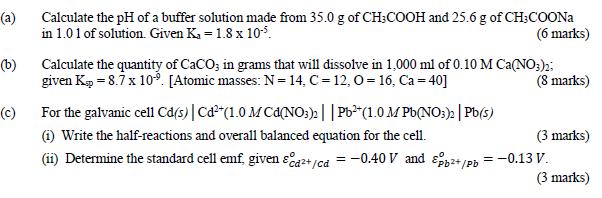
(a) Calculate the pH of a buffer solution made from 35.0 g of CH3COOH and 25.6 g of CH3COONa in 1.01 of solution. Given Ka = 1.8 x 10-5. (6 marks) (b) Calculate the quantity of CaCO; in grams that will dissolve in 1,000 ml of 0.10 M Ca(NO3)2; given Kp = 8.7 x 109. [Atomic masses: N = 14, C=12, O=16, Ca = 40] (8 marks) (c) For the galvanic cell Cd(s) | Cd+(1.0 M Cd(NO3)2 || Pb (1.0 M Pb(NO3)2 | Pb(s) (i) Write the half-reactions and overall balanced equation for the cell. (3 marks) (ii) Determine the standard cell emf, given &a+/cd = -0.40 V and pp+/pb = -0.13 V. (3 marks)
Step by Step Solution
There are 3 Steps involved in it
the ... View full answer

Get step-by-step solutions from verified subject matter experts


