Question: (a) (b) (c) Write the equilibrium reaction and expression for the solubility product for Ca3(PO4)2. (2 marks) With initial concentrations of [H2]0 = 0.86
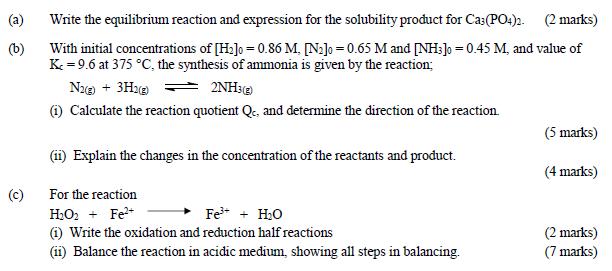
(a) (b) (c) Write the equilibrium reaction and expression for the solubility product for Ca3(PO4)2. (2 marks) With initial concentrations of [H2]0 = 0.86 M. [N2]0 = 0.65 M and [NH3] = 0.45 M, and value of K=9.6 at 375 C, the synthesis of ammonia is given by the reaction; N2(g) + 3H2(g) 2NH3(g) (i) Calculate the reaction quotient Qc, and determine the direction of the reaction. (ii) Explain the changes in the concentration of the reactants and product. For the reaction (5 marks) (4 marks) HO2 + Fe+ Fe + HO (i) Write the oxidation and reduction half reactions (ii) Balance the reaction in acidic medium, showing all steps in balancing. (2 marks) (7 marks)
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
The ... View full answer

Get step-by-step solutions from verified subject matter experts


