Question: a. Can the C-C single bonds in the molecules below fully rotate? Explain why or why not for each. H2NCCNH2 b. Which alkene would you
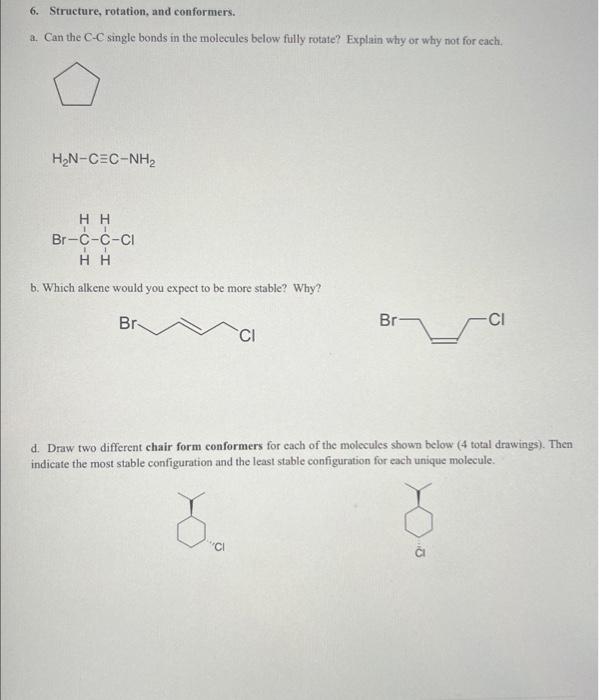
a. Can the C-C single bonds in the molecules below fully rotate? Explain why or why not for each. H2NCCNH2 b. Which alkene would you expect to be more stable? Why? d. Draw two different chair form conformers for each of the molecules shown below ( 4 total drawings). Then indicate the most stable configuration and the least stable configuration for each unique molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


