All chemists know that benzene is unusually stable, that is, it is aromatic. They are also well
Question:
Assigning a value to aromatic stabilization is actually quite straightforward. Consider a hypothetical reaction in which a molecule of hydrogen is added to benzene to yield 1, 3-cyclohexadiene. Next, consider analogous hydrogenation reactions of 1, 3-cyclohexadiene (leading to cyclohexene) and of cyclohexene (leading to cyclohexane):
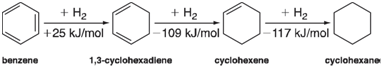
Addition of H2 to benzene trades an H€•H bond and a C€•C Ï€ bond for two C€•H bonds, but in so doing destroys the aromaticity, whereas H2 addition to either 1, 3-cyclohexadiene or cyclohexene trades the same bonds but does not result in any loss of aromaticity (there is nothing to lose). Therefore, the difference in the heats of hydrogenation (134 kJ/mol referenced to 1, 3-cyclohexadiene and 142 kJ/mol referenced to cyclohexene) is a measure of the aromaticity of benzene.
Reliable quantitative comparisons require accurate experimental data (heats of formation). These will generally be available only for very simple molecules and will almost never be available for novel interesting compounds. As a case in point, consider to what extent, if any, the 10 π -electron molecule 1,6-methanocyclodeca-1,3,5,7,9-pentaene (bridged naphthalene), is stabilized by aromaticity. Evidence provided by the X-ray crystal structure suggests a fully delocalized π system. The 10 carbons that make up the base are very nearly coplanar and all C~C bonds are intermediate in length between normal single and double linkages, just as they are in naphthalene.

Calculations provide a viable alternative to experiment for thermo-chemical data. Although absolute hydrogenation energies may be difficult to describe with currently practical models, hydrogenation energies relative to a closely related standard compound are much easier to accurately describe. In this case, the natural standard is benzene.
a. Optimize the geometries of benzene, 1, 3-cyclohexadiene, naphthalene, and 1, 2-dihydronaphthalene using the HF/6-31G* model. Evaluate the energy of the following reaction, relating the energy of hydrogenation of naphthalene to that of benzene (as a standard):
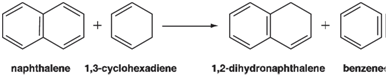
b. Optimize the geometries of 1, 6-methanocyclodeca-1, 3, 5, 7, 9-pentaene and its hydrogenation product using the HF/6-31G* model. Evaluate the energy of hydrogenation relative to that of naphthalene. On the basis of relative hydrogenation energies, would you say that the bridged naphthalene is stabilized to about the same extent as is naphthalene or to a lesser or greater extent? Try to explain your result.
Step by Step Answer:






