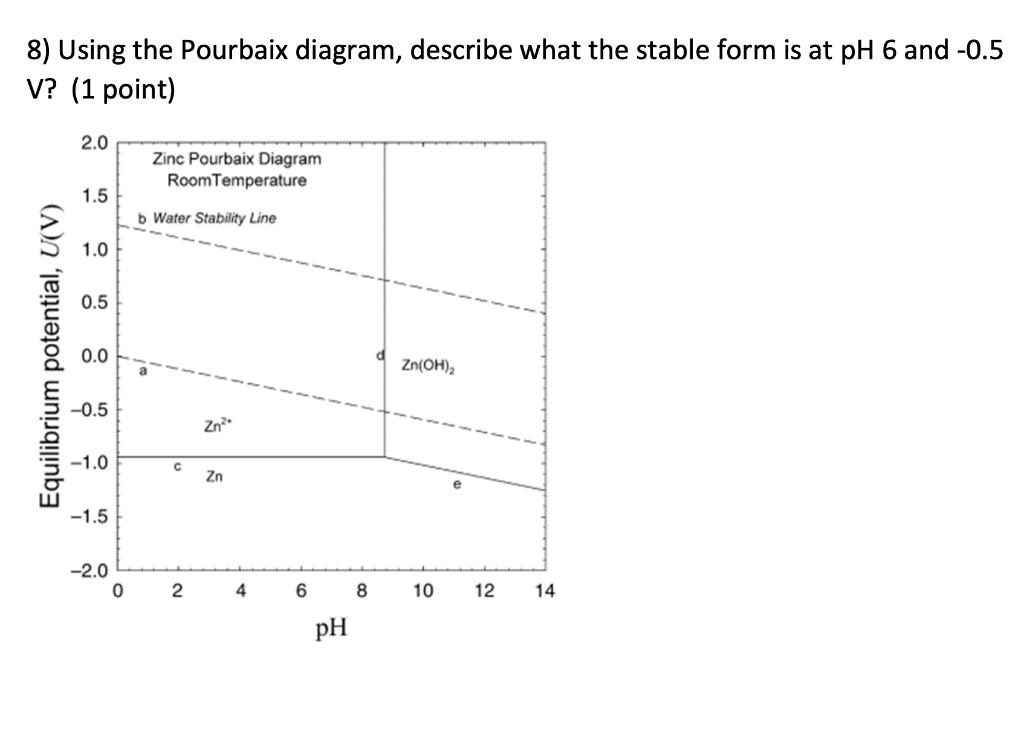
Question: a) Can Zn be easily electroplated? e) Derive the equation that represents line e. 8) Using the Pourbaix diagram, describe what the stable form is
a) Can Zn be easily electroplated?
e) Derive the equation that represents line e.
8) Using the Pourbaix diagram, describe what the stable form is at pH 6 and -0.5 V? (1 point) 2.0 Zinc Pourbaix Diagram Room Temperature 1.5 b Water Stability Line 1.0 0.5 Equilibrium potential, U(V) 0.0 a Zn(OH)2 -0.5 Zn? -1.0 c Zn -1.5 -2.0 0 N 4 6 8 10 12 14 pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


