Question: A 50.0mL solution is initially 1.52%MgCl2 by mass and has a density of 1.05g/mL. What is the freezing point of the solution after you add

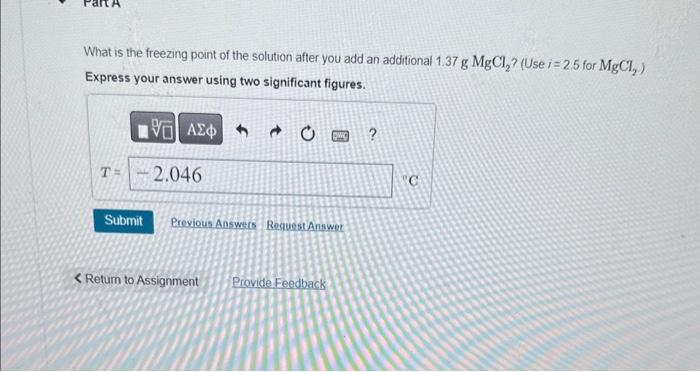
A 50.0mL solution is initially 1.52%MgCl2 by mass and has a density of 1.05g/mL. What is the freezing point of the solution after you add an additional 1.37gMgCl2 ? (Use i=2.5 for MgCl2 ) Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


