Question: A certain gas is compressed from 7.2 me to 2.6 m'. Initial pressure is standard atmospheric pressure. Final pressure is 450 kpa(g). What is the


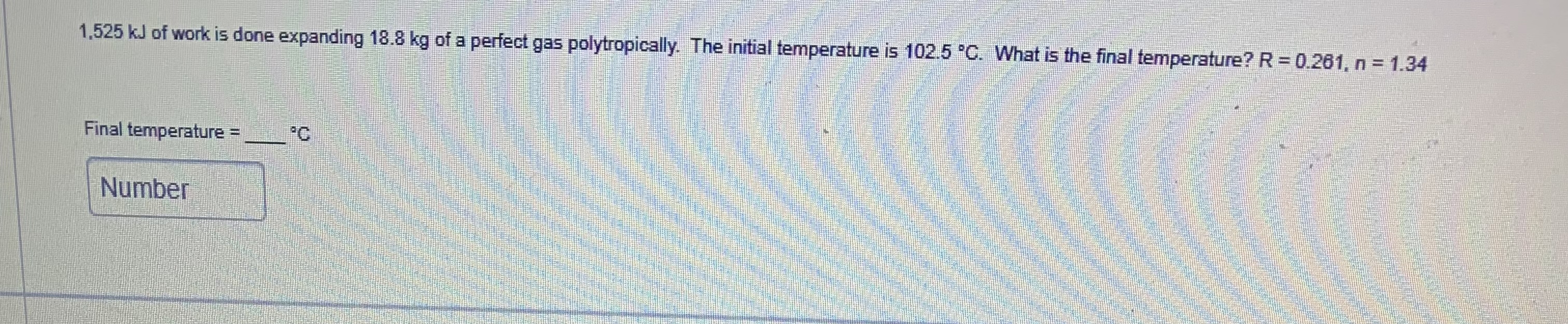
A certain gas is compressed from 7.2 me to 2.6 m'. Initial pressure is standard atmospheric pressure. Final pressure is 450 kpa(g). What is the index of compression for this gas? NumberAn air compressor has a stroke of 0.225 m and a diameter of 0.115 m. It takes in a gas at 1 bar(9) and compresses it to a pressure of 14.5 bar(g). The compression ratio is 3:1. If compression follows PV"=constant. What is "n"? Assume standard atmospheric pressure. Number1.525 kJ of work is done expanding 18.8 kg of a perfect gas polytropically. The initial temperature is 102.5 .C. What is the final temperature? R = 0.261, n = 1.34 Final temperature = Number
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


