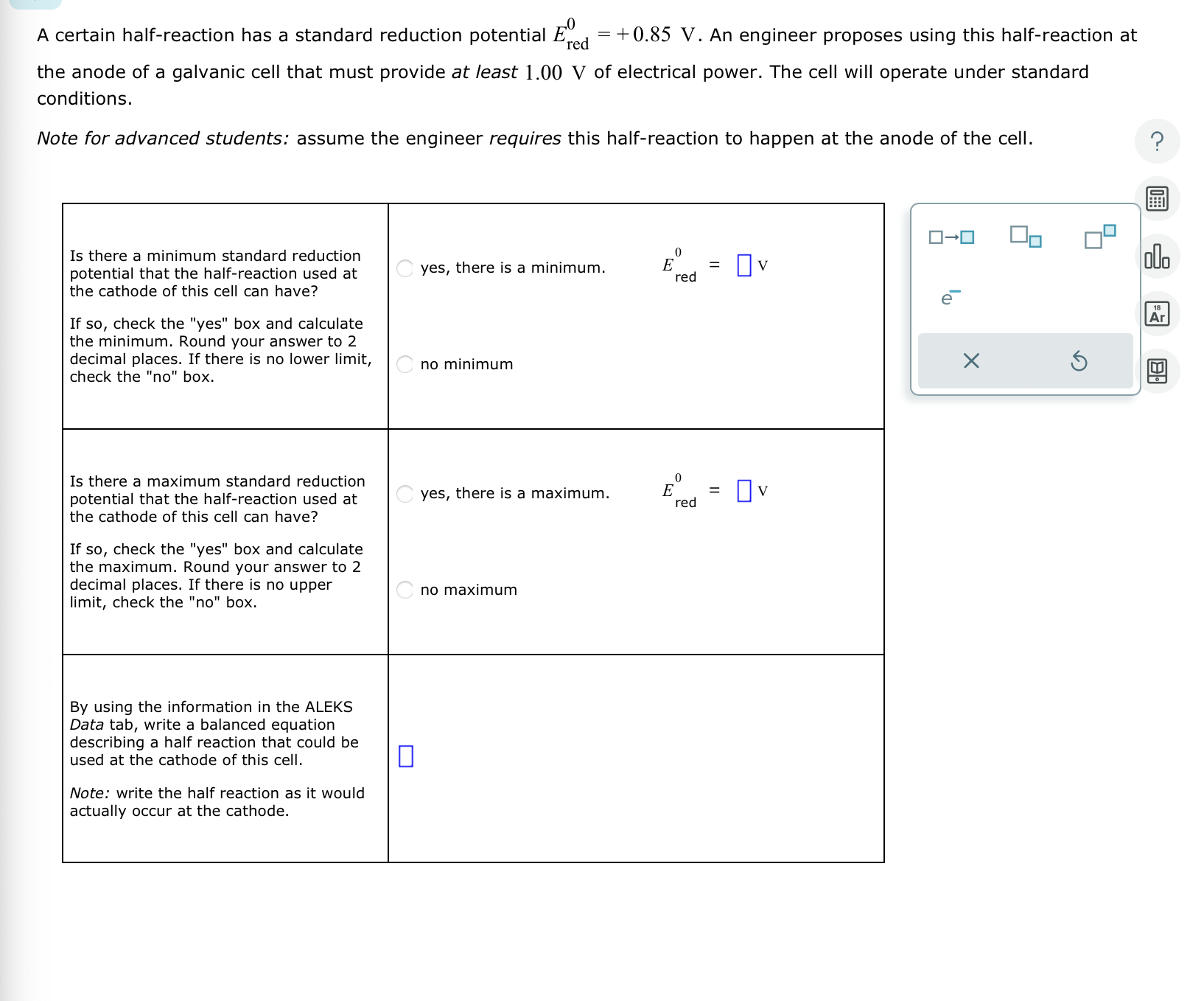
Question: A certain half - reaction has a standard reduction potential E r e d 0 = + 0 . 8 5 V . An engineer
A certain halfreaction has a standard reduction potential An engineer proposes using this halfreaction at
the anode of a galvanic cell that must provide at least of electrical power. The cell will operate under standard
conditions.
Note for advanced students: assume the engineer requires this halfreaction to happen at the anode of the cell.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


