Question: A certain reaction occurred in two steps with the following energies of activation, all in kJ/mol : Step 1. forward: 60 Step 1, backward: x
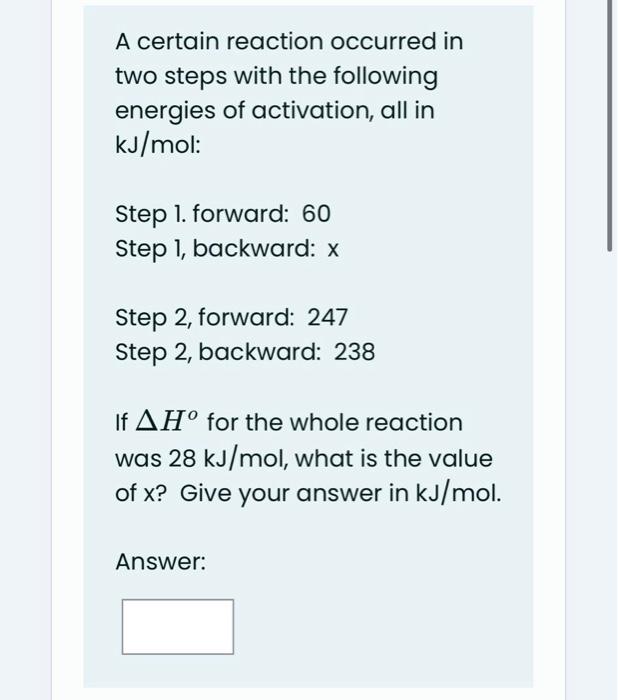
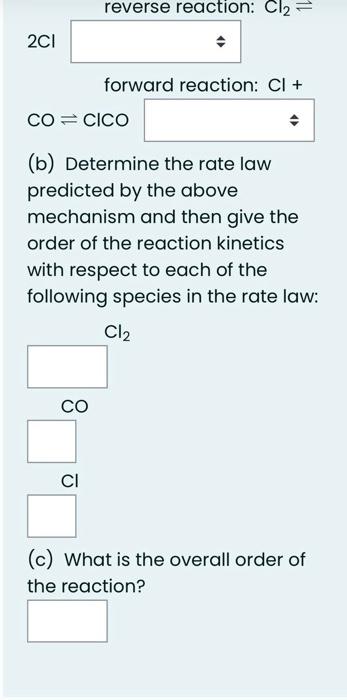
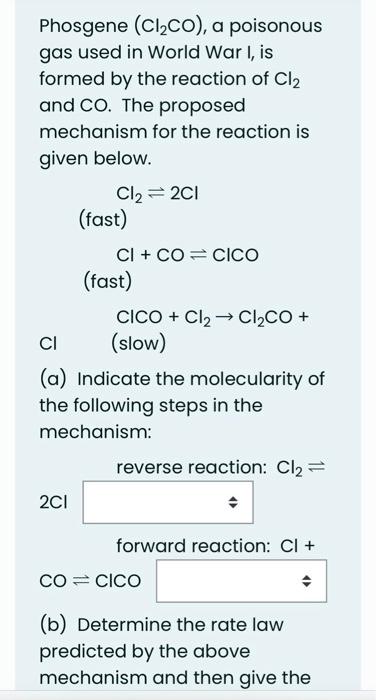
A certain reaction occurred in two steps with the following energies of activation, all in kJ/mol : Step 1. forward: 60 Step 1, backward: x Step 2, forward: 247 Step 2, backward: 238 If Ho for the whole reaction was 28kJ/mol, what is the value of x ? Give your answer in kJ/mol. Answer: reverse reaction: Cl2 2Cl forward reaction: Cl+ COClCO (b) Determine the rate law predicted by the above mechanism and then give the order of the reaction kinetics with respect to each of the following species in the rate law: Cl2 CO Cl (c) What is the overall order of the reaction? Phosgene (Cl2CO), a poisonous gas used in World War I, is formed by the reaction of Cl2 and CO. The proposed mechanism for the reaction is given below. Cl22Cl (fast) Cl+COClCO (fast) ClCO+Cl2Cl2CO+ (slow) (a) Indicate the molecularity of the following steps in the mechanism: reverse reaction: Cl2 2Cl forward reaction: Cl+ COClCO (b) Determine the rate law predicted by the above mechanism and then give the
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


