Question: A chemical engineering intern in Thuthuka Separations Ltd is assigned to set up a pilot distillation plant that will separate a mixture of 0.19 mole
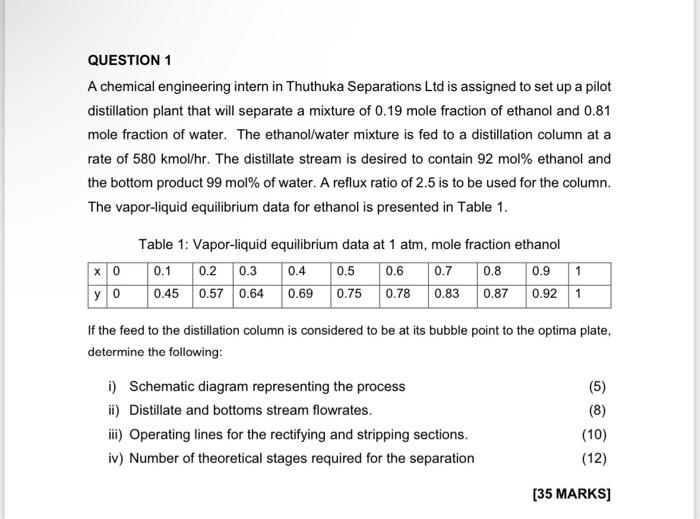

A chemical engineering intern in Thuthuka Separations Ltd is assigned to set up a pilot distillation plant that will separate a mixture of 0.19 mole fraction of ethanol and 0.81 mole fraction of water. The ethanol/water mixture is fed to a distillation column at a rate of 580kmol/hr. The distillate stream is desired to contain 92mol% ethanol and the bottom product 99mol% of water. A reflux ratio of 2.5 is to be used for the column. The vapor-liquid equilibrium data for ethanol is presented in Table 1. Table 1: Vapor-liquid equilibrium data at 1atm, mole fraction ethanol If the feed to the distillation column is considered to be at its bubble point to the optima plate, determine the following: i) Schematic diagram representing the process (5) ii) Distillate and bottoms stream flowrates. (8) iii) Operating lines for the rectifying and stripping sections. iv) Number of theoretical stages required for the separation 1. Gaseous feed with reactants A and B(vo=20m3/hr) passes through an experimental reactor packed with catalyst of a weight 8kg Find the conversion of the reactants if the feed contains CAO=0,2mol/m3 and CBO= 20mol/m3 The reaction occurs as follows: A+BR+SrA=1,2CACBkghrmol 2. For a packed bed reactor, how much catalyst is needed to achieve 85% conversion of 1000m3/hr of pure gaseous A. The inlet concentration of A is 1000mol/m3 and the rate and stoichiometry are given by: ARrA=1+0,02cA50cAkghrmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


