Question: A closed system changed from state a to state b via the path a+c+b, as shown in Fig. 1. It was found that 450 J
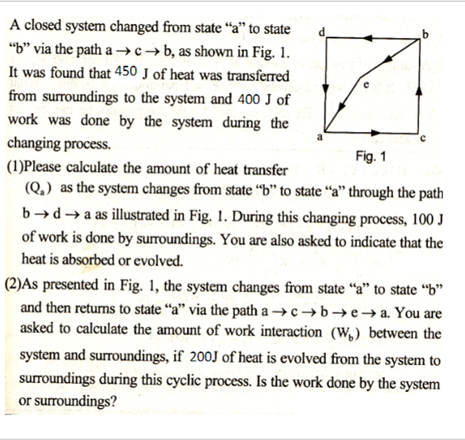
A closed system changed from state a to state b via the path a+c+b, as shown in Fig. 1. It was found that 450 J of heat was transferred from surroundings to the system and 400 J of work was done by the system during the changing process. Fig. 1 (1)Please calculate the amount of heat transfer (Q.) as the system changes from state b to state a through the path b+d+a as illustrated in Fig. 1. During this changing process, 100 J of work is done by surroundings. You are also asked to indicate that the heat is absorbed or evolved. (2)As presented in Fig. 1, the system changes from state a to state b and then returns to state a via the path a a. You are asked to calculate the amount of work interaction (W) between the system and surroundings, if 200J of heat is evolved from the system to surroundings during this cyclic process. Is the work done by the system or surroundings
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


