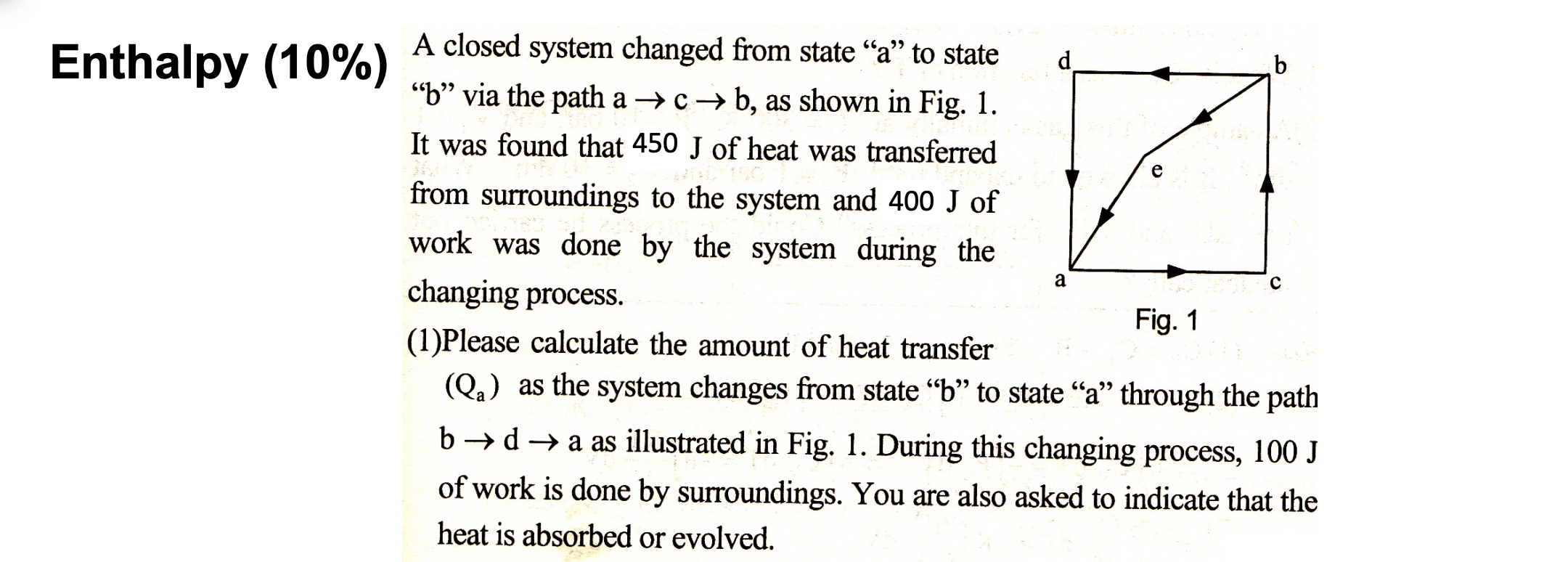
Question: please answer this question Enthalpy (10%) d b a A closed system changed from state a to state b via the path a b, as
please answer this question
Enthalpy (10%) d b a A closed system changed from state a to state b via the path a b, as shown in Fig. 1. It was found that 450 J of heat was transferred from surroundings to the system and 400 J of work was done by the system during the changing process. (1)Please calculate the amount of heat transfer (Qa) as the system changes from state b to state a through the path b + d + a as illustrated in Fig. 1. During this changing process, 100 J of work is done by surroundings. You are also asked to indicate that the heat is absorbed or evolved. Fig. 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


