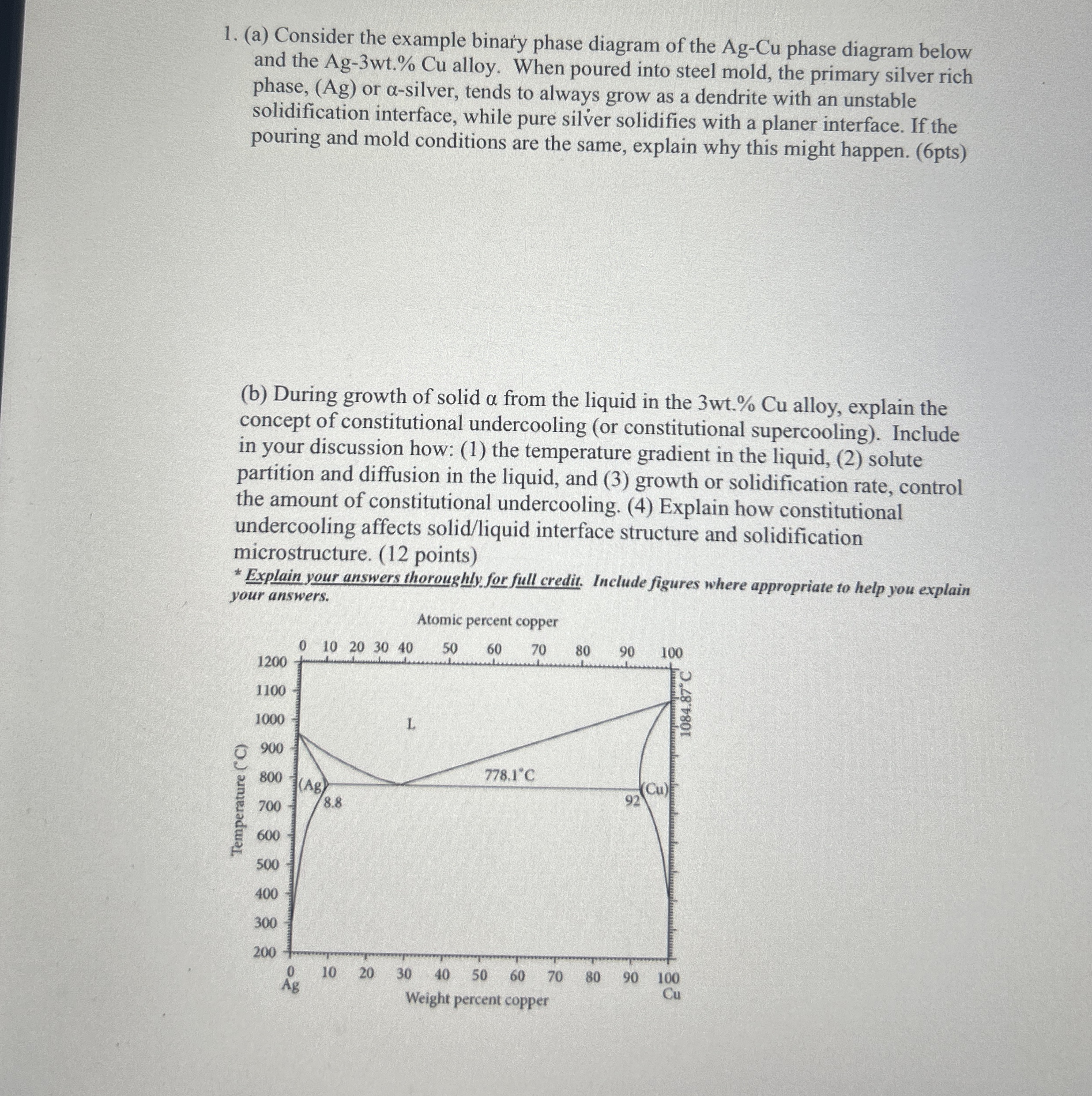
Question: ( a ) Consider the example binary phase diagram of the A g - C u phase diagram below and the A g - 3
Step by Step Solution
There are 3 Steps involved in it
Answer to a Why dendritic growth occurs for Ag3wtCu alloy In the Ag3wtCu alloy the solidifying phase is a silverrich phase that contains a small amount of Cu When solidification starts the phase rejec... View full answer

Get step-by-step solutions from verified subject matter experts


