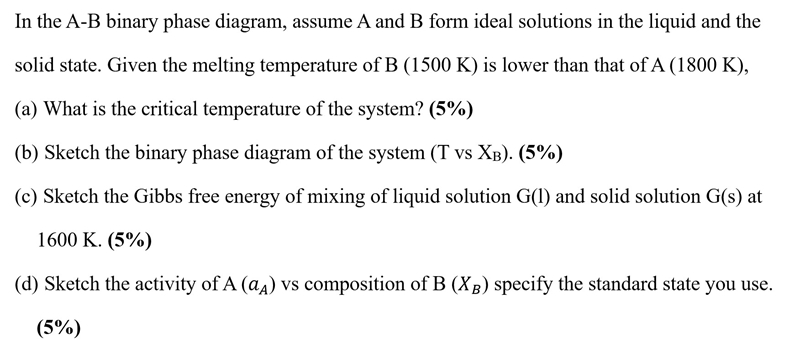
Question: In the A - B binary phase diagram, assume A and B form ideal solutions in the liquid and the solid state. Given the melting
In the binary phase diagram, assume A and form ideal solutions in the liquid and the
solid state. Given the melting temperature of B is lower than that of
a What is the critical temperature of the system?
b Sketch the binary phase diagram of the system vs
c Sketch the Gibbs free energy of mixing of liquid solution and solid solution at
K
d Sketch the activity of vs composition of specify the standard state you use.
In the AB binary phase diagram, assume A and B form ideal solutions in the liquid and the
solid state. Given the melting temperature of B K is lower than that of A K
a What is the critical temperature of the system?
b Sketch the binary phase diagram of the system T vs Xb
c Sketch the Gibbs free energy of mixing of liquid solution Gl and solid solution Gs at
K
d Sketch the activity of A aA vs composition of B XB specify the standard state you use.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


